
93
.pdf
Тулеуханов С.Т. және т.б.
Perez-Reyes E. G protein-mediated inhibition of Cav3.2 T-type channels revisited. // Mol. Pharmacol. – 2010. – Vol. 77, No 2.
– P. 136-138.
Sánchez-Alonso J.L., Halliwell J.V., Colino A. ZD 7288 inhibits T-type calcium current in rat hippocampal pyramidal cells. // Neurosci Lett. – 2008. – Vol. 439, No 3. – P. 275-280.
Iyer R., Ungless M.A., Faisal A.A. Calcium-activated SK channels control firing regularity by modulating sodium channel availability in midbrain dopamine neurons. // Sci Rep. – 2017. – Vol. 7, No 1. – P. 5248.
Rehak R., Bartoletti T.M., Engbers J.D., Berecki G., Turner R.W., Zamponi G.W. Low voltage activation of KCa1.1 current by Cav3-KCa1.1 complexes. // PLoS One. – 2013. – Vol. 8, No 4. – P. 618.
Wolfart J., Roeper J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. // J Neurosci. – 2002. – Vol. 22, No 9. – P. 3404-3413.
Xu J., Clancy C.E. Ionic mechanisms of endogenous bursting in CA3 hippocampal pyramidal neurons: a model study. // PloS One. – 2008. – Vol. 3, No 4. – P. 2056.
Wolfe J.T., Wang H., Perez-Reyes E., Barrett P.Q. Stimulation of recombinant Ca(v)3.2, T-type, Ca(2+) channel currents by CaMKIIgamma(C). // J. Physiol. – 2002. – Vol. 538, No 2. – P. 343-355.
Chemin J., Mezghrani A., Bidaud I., Dupasquier S., Marger F., Barrère C., Nargeot J., Lory P. Temperature-dependent modulation of CaV3 T-type calcium channels by protein kinases C and A in mammalian cells. // J Biol Chem. – 2007. – Vol. 282, No 45.
– P. 310-318.
Chemin J., Monteil A., Perez-Reyes E., Nargeot J., Lory P. Direct inhibition of T-type calcium channels by the endogenous cannabinoid anandamide. // EMBO J. – 2001. – Vol. 20, No 24. – P. 7033-7040.
Chemin J., Nargeot J., Lory P. Chemical determinants involved in anandamide-induced inhibition of T-type calcium channels. // J Biol Chem. – 2007. – Vol. 282, No 4. – P. 2314-2323.
Talavera K., Staes M., Janssens A., Droogmans G., Nilius B. Mechanism of arachidonic acid modulation of the T-type Ca2+ channel alpha1G. // J Gen Physiol. – 2004. – Vol. 124, No 3. – P. 225-238.
Zhang Y., Cribbs L.L., Satin J. Arachidonic acid modulation of alpha1H, a cloned human T-type calcium channel. // Am J Physiol Heart Circ Physiol. – 2000. – Vol. 278, No 1. – P. 184-193.
Wolfe J.T., Wang H., Howard J., Garrison J.C., Barrett P. Q. T-type calcium channel regulation by specific G-protein betagamma subunits. // Nature. – 2003. – Vol. 424, No 6945. – P. 209-213.
Tao J., Hildebrand M.E., Liao P., Liang M.C., Tan G., Li S., Snutch T.P., Soong T.W. Activation of corticotropin-releasing factor receptor 1 selectively inhibits CaV3.2 T-type calcium channels. // Mol Pharmacol. – 2008. – Vol. 73, No 6. – P. 1596-1609.
HildebrandM.E., David L.S., HamidJ., MulatzK.,GarciaE., ZamponiG. W,SnutchT.P. Selectiveinhibitionof Cav3.3 T-type calcium channels by Galphaq/11-coupled muscarinic acetylcholine receptors. // J Biol Chem. – 2007. – Vol. 282, No 29. – P. 243255.
Nelson M.T., Todorovic S.M., Perez-Reyes E. The role of T-type calcium channels in epilepsy and pain. // Curr Pharm Des. – 2006. – Vol. 12, No 18. – P. 2189-2197.
Todorovic S.M., Jevtovic-Todorovic V. Targeting of CaV3.2 T-type calcium channels in peripheral sensory neurons for the treatment of painful diabetic neuropathy. // Pflügers Arch. – 2014. – Vol. 466, No 4. – P. 701-706.
Kopecky B.J., Liang R., Bao J. T-type calcium channel blockers as neuroprotective agents. // J Physiol. – 2014. – Vol. 466, No 4. – P. 757.
Orestes P., Bojadzic D., Chow R. M., Todorovic S.M. Mechanisms and functional significance of inhibition of neuronal T-type calcium channels by isoflurane. // Mol Pharmacol. – 2009. – Vol. 75, No 3. – P. 542-554.
Spitzer N.C., Olson E., Gu X. Spontaneous calcium transients regulate neuronal plasticity in developing neurons. // Neurobiol.
– 1995. – Vol. 26, No 3. – P. 316-324.
Robinson H.P., Kawahara M., Jimbo Y., Torimitsu K., Kuroda Y., Kawana A. Periodic synchronized bursting and intracellular calcium transients elicited by low magnesium in cultured cortical neurons. // J Neurophysiol. – 1993. – Vol. 70, No 4. – P. 16061616.
Guzman J.N., Sánchez-Padilla J., Chan C.S., Surmeier D.J. Robust pacemaking in substantia nigra dopaminergic neurons. // J Neurosci. – 2009. – Vol. 29, No 35. – P. 111-119.
Surmeier D.J., Schumacker P.T. Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease. // J Biol Chem. – 2013. – Vol. 288, No 15. – P. 736-741.
Lieberman O.J., Choi S.J., Kanter E., Saverchenko A., Frier M.D., Fiore G.M., Wu M., Kondapalli J., Zampese E., Surmeier D.J., Sulzer D., Mosharov E.V. α-Synuclein-Dependent Calcium Entry Underlies Differential Sensitivity of Cultured SN and VTA Dopaminergic Neurons to a Parkinsonian Neurotoxin. // eNeuro. – 2017. – Vol. 4, No 6. – P. 167-170.
Surmeier D.J., Obeso J.A., Halliday G.M. Selective neuronal vulnerability in Parkinson disease. // Neurosci. – 2017. – Vol. 18, No 2. – P. 101-113.
Chan C.S., Guzman J.N., Ilijic E., Mercer J.N., Rick C., Tkatch T., Meredith G.E., Surmeier D.J. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. // Nature. – 2007. – Vol. 447, No 7148. – P. 1081-1086.
Grynkiewicz G., Poenie M., Tsien R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. // J Biol Chem. – 1985. – Vol. 260, No 6. – P. 3440-3450.
Hayashi H., Miyata H., Terada H., Satoh H., Katoh H., Nakamura T., Kobayashi A. Ca2+ waves and intracellular Ca2+ concentration in guinea pig and rat myocytes. // Jpn Heart J. – 1994. – Vol. 35, No 5. – P. 673-682.
Колягин В.В. Эпилепсия // Иркутск: РИО ГБОУ ДПО ИГМАПО. – 2013. – № 1. – С. 21-23.
ISSN 1563-034Х |
Eurasian Journal of Ecology. №1 (58). 2019 |
81 |
еISSN 2617-7358 |
|
|

ГАМҚ(А)- және глутаматты рецепторлардың баяу полярсыздануының ауытқу шегінің өзгеруі
Nikonenko I., Bancila M., Bloc A., Muller D., Bijlenga P. Inhibition of T-type calcium channels protects neurons from delayed ischemia-induced damage. // Mol Pharmacol. – 2005. – Vol. 68, No 1. – P. 84-89.
Kononov A.V., Ball N.V., Zinchenko V.P., Biochemistry (Moscow). // Suppl. Ser. A: Membrane and Cell Biology. – 2011. – Vol. 5, No 2. – P. 162.
Chevalier M., Lory P., Mironneau C., Macrez N., Quignard J.F. (2006) T-type CaV3.3 calcium channels produce spontaneous low-threshold action potentials and intracellular calcium oscillations. // Eur J Neurosci. – 2006. – Vol. 23, No 9. – P. 2321-2329.
CooperD.C.Thesignificanceofactionpotentialburstinginthebrainrewardcircuit.//Neurochem.–2002.–Vol.41,No5.–P. 333-340.
Swadlow H.A., Gusev A.G., Bezdudnaya T. Activation of a cortical column by a thalamocortical impulse. // J Neurosci. – 2002.
– Vol. 22, No 17. – P. 7766-7773.
Zinchenko V.P., Turovsky E.A., Turovskaya M.V., et al. Biochemistry (Moscow). // Suppl. Ser. A: Membrane and Cell Biology. – 2016. – Vol. 10, No 2. – P. 118.
Zinchenko V.P., Gaidin S.G., Teplov I.Y., Kosenkov A.M.. Biochemistry (Moscow). // Suppl. Ser. A: Membrane and Cell Biology. – 2017. – Vol. 11, No 4. – P. 261.
References
Chan C.S., Guzman J.N., IlijicE., Mercer J.N., Rick C., Tkatch T., Meredith G.E., Surmeier D.J. (2007) ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature., vol. 447, no 7148, p. 1081-1086.
Chemin J., Mezghrani A., Bidaud I., Dupasquier S., Marger F., Barrère C., Nargeot J., Lory P. (2007) Temperature-dependent modulation of CaV3 T-type calcium channels by protein kinases C and A in mammalian cells. J Biol Chem., vol. 282, no 45, p. 310-318.
Chemin J., Monteil A., Perez-Reyes E., Nargeot J., Lory P. (2001) Direct inhibition of T-type calcium channels by the endogenous cannabinoid anandamide. EMBO J. vol. 20, no 24, p. 7033-7040.
Chemin J., Nargeot J., Lory P. (2007) Chemical determinants involved in anandamide-induced inhibition of T-type calcium channels. J Biol Chem., vol. 282, no 4, p. 2314-2323.
Chevalier M., Lory P., Mironneau C., Macrez N., Quignard J.F. (2006) T-type CaV3.3 calcium channels produce spontaneous low-threshold action potentials and intracellular calcium oscillations. Eur J Neurosci., vol. 23, no 9, p. 2321-2329.
Cooper D.C. (2002) The significance of action potential bursting in the brain reward circuit. Neurochem., vol. 41, no 5, p. 333-
340.
GrynkiewiczG.,PoenieM.,TsienR.Y.(1985)AnewgenerationofCa2+indicatorswithgreatlyimprovedfluorescenceproperties. J Biol Chem., vol. 260, no 6, p. 3440-3450.
Guzman J.N., Sánchez-Padilla J., Chan C.S., Surmeier D.J. (2009) Robust pacemaking in substantia nigra dopaminergic neurons. J Neurosci., vol. 29, no 35, p. 111-119.
Hayashi H., Miyata H., Terada H., Satoh H., Katoh H., Nakamura T., Kobayashi A. (1994) Ca2+ waves and intracellular Ca2+ concentration in guinea pig and rat myocytes. Jpn Heart J., vol. 35, no 5, p. 673-682.
HildebrandM.E., David L.S.,HamidJ., MulatzK., GarciaE., ZamponiG. W, Snutch T.P. (2007)Selectiveinhibitionof Cav3.3 T-type calcium channels by Galphaq/11-coupled muscarinic acetylcholine receptors. J Biol Chem., vol. 282, no 29, p. 243-255.
Huc S., Monteil A., Bidaud I., Barbara G., Chemin J., Lory P. (2009) Regulation of T-type calcium channels: signalling pathways and functional implications. Biochim Biophys Acta. vol. 1793, no 6, p. 947-952.
Iyer R., Ungless M.A., Faisal A.A. (2017) Calcium-activated SK channels control firing regularity by modulating sodium channel availability in midbrain dopamine neurons. Sci Rep., vol. 7, no 1, p. 5248.
Kim D., Song I., Keum S., Lee T., Jeong M.J., Kim S.S, Mc Enery M.W., Shin H.S. (2001) Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca(2+) channels. Neuron., vol. 31, no 1, p. 35-45.
Kolyagin V.V. (2013) Epilepsiya [Epilepsy]. Irkutsk: RIO GBOU DPO IGMAPO, vol 1, pp. 21-23.
Kononov A.V., Ball N.V., Zinchenko V.P. (2011) Biochemistry (Moscow). Suppl. Ser. A: Membrane and Cell Biology., vol. 5, no 2, p. 162.
Kopecky B.J., Liang R., Bao J. (2014) T-type calcium channel blockers as neuroprotective agents. J Physiol., vol. 466, no 4, p. 757.
Lieberman O.J., Choi S.J., Kanter E., Saverchenko A., Frier M.D., Fiore G.M., Wu M., Kondapalli J., Zampese E., Surmeier D.J., Sulzer D., Mosharov E.V. (2017) α-Synuclein-Dependent Calcium Entry Underlies Differential Sensitivity of Cultured SN and VTA Dopaminergic Neurons to a Parkinsonian Neurotoxin. eNeuro., vol. 4, no 6, p. 167-170.
Nelson M.T., Todorovic S.M., Perez-Reyes E. (2006) The role of T-type calcium channels in epilepsy and pain. Curr Pharm Des., vol. 12, no 18. p. 2189-2197.
Nikonenko I., Bancila M., Bloc A., Muller D., Bijlenga P. (2005) Inhibition of T-type calcium channels protects neurons from delayed ischemia-induced damage. Mol Pharmacol., vol. 68, no 1, p. 84-89.
Nilius, B., Talavera, K., Verkhratsky, A. (2006) T-type calcium channels: the never ending story. Cell Calcium., vol. 40, no 2, p. 81-88.
Orestes P., Bojadzic D., Chow R. M., Todorovic S.M. (2009) Mechanisms and functional significance of inhibition of neuronal T-type calcium channels by isoflurane. Mol Pharmacol., vol. 75, no 3, p. 542-554.
82 |
Хабаршы. Экология сериясы. №1 (58). 2019 |

Тулеуханов С.Т. және т.б.
Perez-Reyes E. (2010) G protein-mediated inhibition of Cav3.2 T-type channels revisited. Mol. Pharmacol., vol. 77, no 2, p. 136-138.
Proft J., Weiss N. (2015) G protein regulation of neuronal calcium channels: back to the future. Mol. Pharmacol., vol. 87, no 6, p. 890-906.
Rehak R., Bartoletti T.M., Engbers J.D., Berecki G., Turner R.W., Zamponi G.W. ( 2013) Low voltage activation of KCa1.1 current by Cav3-KCa1.1 complexes. PLoS One., vol. 8, no 4, p. 618.
Robinson H.P., Kawahara M., Jimbo Y., Torimitsu K., Kuroda Y., Kawana A. (1993) Periodic synchronized bursting and intracellular calcium transients elicited by low magnesium in cultured cortical neurons. J Neurophysiol., vol. 70, no 4, p. 1606-1616.
Sánchez-Alonso J.L., Halliwell J.V., Colino A. (2008) ZD 7288 inhibits T-type calcium current in rat hippocampal pyramidal cells. Neurosci Lett., vol. 439, no 3, p. 275-280.
SongI.,KimD.,ChoiS.,SunM.,KimY.,ShinH.S.(2004)Roleofthealpha1GT-typecalciumchannelinspontaneousabsence seizures in mutant mice. J Neurosci., vol. 24, no 22, p. 5249-5257.
Spitzer N.C., Olson E., Gu X. (1995) Spontaneous calcium transients regulate neuronal plasticity in developing neurons. Neurobiol., vol. 26, no 3, p. 316-324.
Surmeier D.J., Obeso J.A., Halliday G.M. (2017) Selective neuronal vulnerability in Parkinson disease. Neurosci., vol. 18, no 2, p. 101-113.
Surmeier D.J., Schumacker P.T. (2013) Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease. J Biol Chem., vol. 288, no 15, p. 736-741.
Swadlow H.A., Gusev A.G., Bezdudnaya T. (2002) Activation of a cortical column by a thalamocortical impulse. J Neurosci., vol. 22, no 17, p. 7766-7773.
Talavera K., Staes M., Janssens A., Droogmans G., Nilius B. (2004) Mechanism of arachidonic acid modulation of the T-type Ca2+ channel alpha1G. J Gen Physiol., vol. 124, no 3, p. 225-238.
Tao J., Hildebrand M.E., Liao P., Liang M.C., Tan G., Li S., Snutch T.P., Soong T.W. (2008) Activation of corticotropinreleasing factor receptor 1 selectively inhibits CaV3.2 T-type calcium channels. Mol Pharmacol., vol. 73, no 6, p. 1596-1609.
Todorovic S.M., Jevtovic-Todorovic V. (2014) Targeting of CaV3.2 T-type calcium channels in peripheral sensory neurons for the treatment of painful diabetic neuropathy. Pflügers Arch., vol. 466, no 4, p. 701-706.
Wolfart J., Roeper J. (2002) Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci., vol. 22, no 9, p. 3404-3413.
Wolfe J.T., Wang H., Howard J., Garrison J.C., Barrett P. Q. (2003) T-type calcium channel regulation by specific G-protein betagamma subunits. Nature., vol. 424, no 6945, p. 209-213.
Wolfe J.T., Wang H., Perez-Reyes E., Barrett P.Q. (2002) Stimulation of recombinant Ca(v)3.2, T-type, Ca(2+) channel currents by CaMKIIgamma(C). J. Physiol., vol. 538, no 2, p. 343-355.
Xu J., Clancy C.E. (2008) Ionic mechanisms of endogenous bursting in CA3 hippocampal pyramidal neurons: a model study. PloS One., vol. 3, no 4, p. 2056.
Zhang Y., Cribbs L.L., Satin J. (2000) Arachidonic acid modulation of alpha1H, a cloned human T-type calcium channel. Am J Physiol Heart Circ Physiol., vol. 278, no 1, p. 184-193.
Zinchenko V.P., Gaidin S.G., Teplov I.Y., Kosenkov A.M.. (2017) Biochemistry (Moscow). Suppl. Ser. A: Membrane and Cell Biology., vol. 11, no 4, p. 261.
Zinchenko V.P., Turovsky E.A., Turovskaya M.V., et al. (2016) Biochemistry (Moscow). Suppl. Ser. A: Membrane and Cell Biology., vol. 10, no 2, p. 118.
ISSN 1563-034Х |
Eurasian Journal of Ecology. №1 (58). 2019 |
83 |
еISSN 2617-7358 |
|
|

3-бөлім
БИОЛОГИЯЛЫҚ АЛУАНТҮРЛІЛІКТІ САҚТАУДЫҢ ӨЗЕКТІ МӘСЕЛЕЛЕРІ
Раздел 3
АКТУАЛЬНЫЕ ПРОБЛЕМЫ СОХРАНЕНИЯ БИОЛОГИЧЕСКОГО РАЗНООБРАЗИЯ
Section 3
ACTUALPROBLEMS
OFBIODIVERSITYCONSERVATION
IRSTI 34.43.35, 61.45.31
Baktybayeva L.K.1, Namaz E.R.2, Kalzhan K.M.2,
Berlin Kennet Darrell3, Umbetiyarova L.B.4
1PhD, associate professor, acting professor of department biophysics and biomedicine, e-mail: layilia.baktybaeva@kaznu.kz
2student of department biophysics and biomedicine,
e-mail: namaz.elmira@gmail.com, e-mail: kalzhan.kassiyet@gmail.com 3PhD, professor, e-mail: kennet.berlin@okstate.edu
4PhD, senior lector of department biophysics and biomedicine, e-mail: lyazzat.umbetiyarova@kaznu.kz 1,2,4 al-Farabi Kazakh National university, Kazakhstan, Almaty
3Center for Health Sciences, USA, Oklahoma City
PHARMACOLOGICAL PROPERTIES
OF ENDEMIC PLANTS GROWING IN THE STEPPES
OF KAZAKHSTAN
The number of people who suffer from secondary immunodeficiency diseases is increasing. Secondary immunodeficiency diseases develop due to environmental degradation, abuse of various preservatives, stabilizers in foods and long-term storage products, abuse of the use of drugs with a cytostatic effect, the use of antibiotics in the cultivation of cattle and poultry. The compoundswere obtained from plants of the Halostachys caspica (Pall) C.A.Mey.ex Schrenk,Suaeda microphylla Pall., Climacoptera obtusifolia (Schrenk) Botsch.Plant extracts were investigatedon myelostimulating activity. The compound obtained from plants of the Halostachys caspica (Pall) C.A.Mey.ex Schrenk by means of water-alcohol extractionwithout heating showed high myelostimulating activity. It effectively stimulated erythro-, thrombocytoand leukopoiesis, and in stimulating the leukocyte population, it equally effectively increased the values of granulocyte and agranulocyte leukocytes. The compounds derived from the Suaeda microphylla Pall. and Climacoptera obtusifolia (Schrenk) Botsch.showed low myelostimulating activity. It should be noted that the water-alcohol extracts of plants without heating showed higher activity than the extracts that have undergone thermal heating.
Key words: myelostimulating activity, endemic plants, steppes of Kazakhstan.
Бақтыбаева Л.Қ.1, Намаз Э.Р.2, Қалжан Қ.М.2, Берлин Кеннет Даррел3, Умбетиярова Л.Б.4
1биология ғылымдарының кандидаты, доцент, биофизики және биомедицина кафедрасының профессоры м.а., e-mail:layla.baktybaeva@kaznu.kz 2студент, e-mail: namaz.elmira@gmail.com, e-mail: kalzhan.kassiyet@gmail.com 3PhD, профессор, e-mail:kennet.berlin@okstate.edu
4медицина ғылымдарының кандидаты, биофизикa және биомедицина кафедрасының аға оқытушысы, e-mail: lyazzat.umbetiyarova@kaznu.kz
1,2,4 әл-Фараби атындағы Қазақ ұлттық университеті, Қазақстан, Алматы қ. 3Оклахома Мемлекеттік университеті, Денсаулық Ғылымдар Орталығы, США, Оклахома қ.
Қазақстанның жазық даласында өсетін эндемик өсімдіктердің фармакологиялық қасиеттері
Экологиялық жағдайдың, пайдаланатын өнімдердің сапасының нашарлауы, әр түрлі цитостатикалық әсері бар дәрілік препараттарды мезгілсіз пайдалану нәтижесінде екінші реттік иммунодефицитке шалдыққан адамдардың саны күрт артуда. Каспийлік соляноколосник, кішіжапырақты сведа және тегісжапырақты климакоптера өсімдіктерінен алынған сығындыларға жүргізілген зерттеу жұмыстарының нәтижесінде миелостимулдаушы әсері ең жоғары қыздырусыз
© 2019 Al-Farabi Kazakh National University

Baktybayeva L.K. et al.
су-спирттік экстракттау әдісімен бөлініп алынған каспийлік соляноколосник өсімдігінің сығындысы екендігі анықталды. Ол эритро-, тромбоцитожәне лейкопоэзды эффективті түрде стимулдады, сонымен қатар лейкоциттердің санын көбейту кезінде ол гранулоцитарлы және агронулоцитарлы лейкоциттердің түзілуін бірдей мөлшерде стимулдай алды. Тегісжапырақты климакоптера өсімдігінен алынған сығындының миелостимулдаушы әсері каспийлік соляноколосник өсімдігіне қарағанда бәсеңдеу болды, алайда кішіжапырақты сведа өсімдігінің сығындысынан стимулдаушы әсерінен белсендірек. Өсімдіктердің қыздырусыз алынған суспирттік экстракттарының белсенділігі термиялық өңдеуден өткен өсімдік сығындыларына қарағанда белсендігі жоғары екендігін атап айтып кеткен жөн.
Түйін сөздер: миелостимулдаушы белсенділік, эндемик өсімдіктер, Қазақстанның аридті аймағы.
Бақтыбаева Л.Қ.1, Намаз Э.Р.2, Қалжан Қ.М.2, Берлин Кеннет Даррел3, Умбетиярова Л.Б.3
1кандидат биологических наук, доцент, и.о. профессора кафедры биофизики и биомедицины, e-mail: layla.baktybaeva@kaznu.kz
2студент, e-mail: namaz.elmira@gmail.com, e-mail: kalzhan.kassiyet@gmail.com 3PhD, профессор, e-mail: kennet.berlin@okstate.edu
4кандидат медицинских наук,
старший преподаватель кафедры биофизики и биомедицины, e-mail: lyazzat.umbetiyarova@kaznu.kz 1,2,4 Казахский национальный университет им. аль-Фараби, Казахстан, г. Алматы
3Оклахомский Государственный университет, Центр наук о здоровье, США, г. Оклахома
Фармакологические свойства растений эндемиков, произрастающих в степях Казахстана
Число людей, страдающих вторичными иммунодефицитными заболеваниями, увеличивается во всем мире. Прирост населения со вторичными иммунодефицитными заболеваниями происходит из-за ухудшения экологической обстановки, использования стабилизаторов, консервантов, красителей в процессе производства продуктов питания длительного хранения, расширением спектра заболеваний с использованием цитостатических препаратов и использованием антибиотиков при выращивании скота и птицы. Были исследованы на миелостимулирующую активность экстракты, полученные из растений эндемиков Казахстана: cоляноколосника прикаспийского, cведы мелколистной и климакоптеры туполистной. Высокую миелостимулирующую активность проявило соединение, полученное из растения cоляноколосника прикаспийского путем водно-спиртового экстрагирования без нагревания. Оно эффективно стимулировало эритро-, тромбоцито- и лейкопоэз, причем в стимуляции лейкоцитарной популяции он одинаково эффективно повышал значения гранулоцитарных и агранулоцитарных лейкоцитов. Соединения, полученные из растения сведы мелколистной, уступали по активности соединениям, полученным из растения соляноколосника прикаспийского, но были активнее соединений, полученных из растения климакоптера туполистная. Следует отметить, что водно-спиртовые экстракты растений без нагревания проявляли активность выше, чем экстракты, прошедшие термическую обработку.
Ключевые слова: миелостимулирующая активность, растения эндемики, аридная зона Казахстана.
Introduction
The number of people who suffer from secondary immunodeficiency diseases is increasing. Secondary immunodeficiency diseases develop due to environmental degradation, abuse of various preservatives, stabilizers in foods and long-term storage products, abuse of the use of drugs with a cytostaticeffect,theuseofantibioticsinthecultivation of cattle and poultry [1]. Various toxic chemical compounds: Sturdart solution, trinitrotoluene, pesticides, especially organochlorine preparations, lindane (gamma-hexachlorocyclohexane) and dichlorodiphenyltrichloroethane also cause
severe pancytopenia. The choice of effective myelostimulating drugs that can restore parameters of peripheral blood in a short period of time is very limited [2]. Thus, pharmacological screening of myelostimulating drugs is relevant. In addition, myelostimulatorsarewidelyusedinophthalmology, surgery, cosmetology and biotechnology.
Materials and Methods
The following plant extracts were received for research: EES.SES/N – 50% water-ethyl alcoholic extract of the plant Halostachys caspica (Pall) C.A.Mey. ex Schrenkwith heating, EES.SEB / N
ISSN 1563-034Х |
Eurasian Journal of Ecology. №1 (58). 2019 |
87 |
еISSN 2617-7358 |
|
|

Pharmacological properties of endemic plants growing in the steppes of Kazakhstan
– 50% water-ethyl alcoholic extract of the plant
Halostachys caspica (Pall) C.A.Mey. ex Schrenk without heating, CIE.SV – 70% water-ethyl alcohol extract plant Suaeda microphylla Pall.without heating, COBE – 70% water-ethyl alcohol extract of Climacoptera obtusifolia (Schrenk) Botsch.with
ethyl extraction of plants
heating, COFL – 70% water-ethyl alcohol extract of plant Climacoptera obtusifolia (Schrenk) Botsch. without heating, COLE – aqueous extract of the plant Climacoptera obtusifolia (Schrenk) Botsch. without heating (Table 1).
Table 1 –Active compounds obtained by water and water-
№ |
Drug code |
Plant |
Chemical composition of plants |
|
|
|
|
|
|
|
EES.SES/N |
Halostachys caspica (Pall) C.A.Mey. |
Flavanoids, free organic acids, amino |
|
1 |
50% water-ethyl alcohol extract of a plant |
|||
|
with heating |
ex Schrenk |
acids, alkaloids, saponins, tannins |
|
|
|
|
||
|
EES.SEB/N |
Halostachys caspica (Pall) C.A.Mey. |
Flavanoids, free organic acids, amino |
|
2 |
50% water-ethyl alcohol extract of a plant |
|||
|
without heating |
ex Schrenk |
acids, alkaloids, saponins, tannins |
|
|
|
|
||
|
|
|
|
|
|
CIE.SV |
|
Flavonoids, free organic acids, amino |
|
3 |
70% water-ethyl alcohol extract of a plant |
Suaeda microphylla Pall. |
||
acids, alkaloids, saponins, coumarins |
||||
|
without heating |
|
||
|
|
|
||
|
СОВЕ |
Climacoptera obtusifolia(Schrenk) |
Flavanoids, free organic acids, amino |
|
4 |
70% water-ethyl alcohol extract of a plant |
|||
Botsch. |
acids, alkaloids, saponins, tannins |
|||
|
without heating |
|
|
|
|
СОLE |
Climacoptera obtusifolia (Schrenk) |
Flavanoids, free organic acids, amino |
|
5 |
Aqueous extract of the plant without |
|||
Botsch. |
acids, alkaloids, saponins, tannins |
|||
|
heating |
|||
|
|
|
||
|
|
|
|
|
|
СОFL |
Climacoptera obtusifolia (Schrenk) |
|
|
6 |
70% water-ethyl alcohol extract of a plant |
Flavanoids, phenolic acids |
||
|
withoutheating |
Botsch. |
|
|
|
|
|
||
|
|
|
|
Healthy, mature animals of laboratory rats of both sexes, 10–15 weeks of age, weighing 210–280 g, were used in the work. The scatter in the groups according to the initial body weight did not exceed ± 10%. The animals were receivedsimultaneously from one nursery – the biological clinic of the Faculty of Biology and Biotechnology of the Al-Farabi Kazakh National University. Before and during the experiment,thecontrolandexperimentalanimalswerekept in the same standard conditions, 6 animals per cage. All types of experiments were carried out in compliance with the chronobiological principles of work andinaccordancewiththe“Rulesforconductingpreclinical (non-clinical) research on biologically active substances”[8].Bloodsamplingwasperformedfrom the orbital vein of rats anesthetized with weak ether anesthesia at 09.00 in the morning. A blood test was performedonahematologicalanalyzerforlaboratory animals“AbacusjuniorVET”(manufacturedbyDiatron, Denmark). The blood leukogramwas monitored by microscopic examination of a smear stained by Romanovsky-Giemsa on an SA3300С microscope for microscopy and digital micrograph under immer-
sion (magnification 7x100) with 500 cells on each smear[6].Myelosuppressionwas induced by administering cytostatic compound benzopyrene at a dose of 30 mg / kg body weight of the animal dissolved in saline in a volume of 0.5 ml three times with an interval of 24 hours [5, 7]. Then, on the 6, 8, 10 day of the observation, once time in day, intramuscularly was injected: to the 1st group – the EEC.SES/N compound at a dose of 5 mg / kg (for all test compounds, physiological saline was the solvent), in a volume of 0.5 ml, The 2nd group – compound EES.SEB/N at a doseof5mg/kginavolumeof0.5ml,the3rdgroup
– compound CIE.SV at a dose of 5 mg / kg in a volume of 0.5 ml, the 4th group – compound COBE in a dose of 5 mg / kg in a volume of 0.5 ml; in group 5, compound COFL in a dose of 5 mg / kg in a volume of 0.5 ml; in group 6, compound COLE in a dose of 5 mg / kg in in volume of 0, 5 ml, the 7th group the drug is compared pantohematogen the dose 0, 4 mg / kginavolumeof0,5ml,8thgroup–placebo(saline) in a volume of 0 5 ml and 9th group of animals was intact. Statistical data processing was performed with casting the Student’s confidence interval.
88 |
Хабаршы. Экология сериясы. №1 (58). 2019 |

Baktybayeva L.K. et al.
Results and Discussion
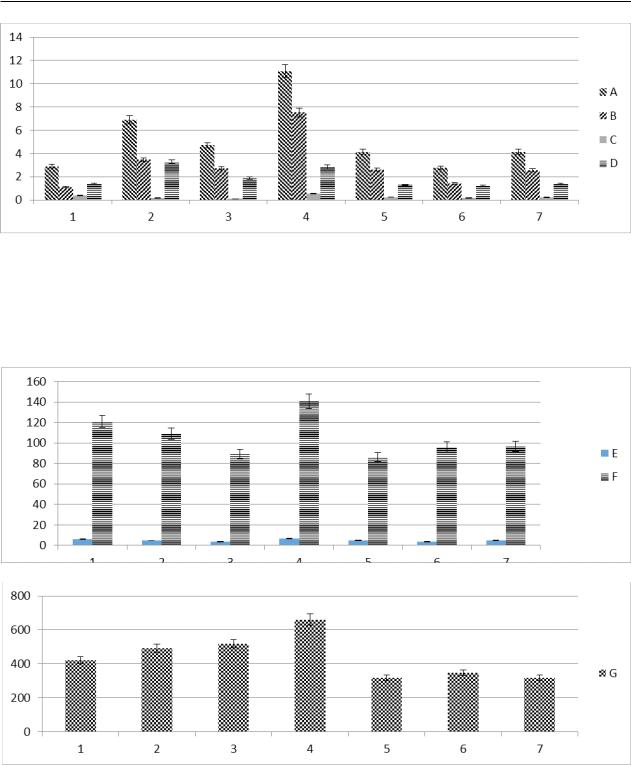
Alreadyonthe1stdayafteradministration,itwas possible to register severe immunosuppression with lesions of leukocyte cells and erythrocyte cells. For control of the level of damage to the blood-forming pools was taken the 3rd day after the administration of the immunosuppressant. After analyzing the hemogram of blood, it was found that the leukocyte pool suffered greatly. The total leukocyte index from the level of intact animals (9.15 ± 1.36) · 109 / L of blood dropped to (2.37 ± 0.16) · 109/L by 3.86 times (p≤0.05). In the leukogram, the absolute and relative values of agranulocytic and granulacytic leukocytes decreased. Among agranulocytes, the absolute values of lymphocytes from (5.46 ± 0.18) · 109/L of blood dropped 3.41 times to (1.60 ± 0.2) · 109/L, while the relative values from index of intact animals (68.03 ± 12.3)% of blood fell only 1.44 times to (47.2 ± 1.8)% of blood. A similar trend was observed with respect to other subpopulations of cells.
Absolute values fell by more than 2 times, and relative values decreased by less than 1.5 times. For example,theabsolutevaluesofmonocyticcellsfrom the value (0.5 ± 0.02) · 109 / L of blood decreased to the value (0.12 ± 0.10) · 109/L of blood, i.e. 4.17 times (p≤0.05), while the relative value (6.28 ± 1.24)%fellonlyto(4.9±1.3)%,whichwasonly1.28 times the difference in the meanings. Granulocyte cells with (3.64 ± 1.22) · 109/L fell to (0.65 ± 0.3) · 109/L, i.e. 5.6 times (p≤0.01). The relative value of granulocytes from (40.0 ± 8.36)% decreased 1.52 times, reaching a value (26.18 ± 4.5)%. In the blood leukogram absolute values are more important in the diagnosis than the relative values of the blood leukogram. Changes in the values of erythrocyte and platelet cells were recorded, but more than 2-fold reduction was not registered. The level of erythrocyte cells (6.5 ± 1.56) · 1012 / L of blood reached the value of (4.93 ± 1.3) · 1012 / L of blood, i.e.adecreasewasobserved1.32times.Hemoglobin levelsfell4.51times.Inintactanimals,itwas(140.7
± 16.7) g / L of blood. After intoxication fell to (90.75 ± 12.0) g / L of blood. But the hematocrit value from the value (39.8 ± 6.3)% fell to 2.88 times (p≤0.05) times, reaching the value (21.21 ± 2.58)%. A critical decrease was observed among platelets. The level of intact animals was (660.25 ±42.3) · 109/L of blood and with an artificially induced immunosuppressive syndrome was (70.5 ± 43.2) · 109/L of blood, which amounted to more than 9.36fold decrease (p≤0.01). Having caused an artificial immunosuppressive syndrome, animals were
treated with new compounds of plant origin. The compoundswereobtainedfromthefollowingplants:
Halostachys caspica (Pall) C.A.Mey.ex Schrenk, Suaeda microphylla Pall., Climacoptera obtusifolia (Schrenk) Botsch. The compounds were obtained by water–ethylandwaterextractionofplants,withand withoutheating.Aftertheintroductionofthestudied compounds, the following results were obtained on the basis of which it is possible to isolate the most active compound EES.ESB/N. This compound was distinguished by a high myelostimulating activity in the series of compounds obtained from other plants and in the series of compounds obtained from the
Halostachys caspica (Pall) C.A.Mey. ex Schrenk. In the group with administration of EEC.SEB/N the level of leukocytes was (6.9 ± 0.4) · 109 / L with a control value (2.79 ± 0.93) · 109/L (p ≤ 0.05) and withavalueatintactanimals(10.4±0.19)·109/L of blood. In the blood leukogram, both in relative and absolutevalues,positivechangeshaveoccurred.The relative value of lymphocytes increased 1.4 times to (70.1 ± 2.3)% against the control value (50.65
± 14.65)% and with the value of intact animals (73.0 ± 3.8)% (Figure 1). More significant changes can be seen in the change in the absolute values of lymphocytes with the indicator (4.8 ± 0.05) · 109 / L opposites tocontrol value (1.6 ± 0.9) · 109/L, which is a 3-fold difference (p ≤0.05) and with the value of intact animals (7.6 ± 0.1) · 109/L(Figure 1). Positive dynamic was observed in the granulocyte leukocyte series, but within 8-10%.
Compound EEC.SEB/H reliably stimulated myelopoiesis of erythrocyte, platelet and leukocyte cell generation. Stimulation of erythropoiesis was effectiveand the level of erythrocytes on the 7th day of observation reached the value (7.43 ± 0.1) · 109/ Lof blood against the control indicator (3.67 ± 0.1) · 109/Lof blood and with the value of intact animals (7.5 ± 0.28) · 109/L(Figure 2). The hemoglobin level reached the value of healthy animals (121.0 ± 1.0) g/L with the control value (96.0 ± 1.0) g/L and the value of intact animals (140.7 ± 4.3) g/L. And the hematocrit level was also very high (37.8 ± 0.91)% against the control value (28.1 ± 0.84)% and the valueofintactanimals(39.8±0.91)%.Therecovery in the leukocyte pool was not as intense as in the platelet(Figure 2). With the value of platelets after administration of benzopyrene (70.5 ± 4.3) · 109 / L, the level of platelets after administration of EEC. ESB/N increased to (691.2 ± 11.0) · 109/Lagainst the control value (447.0 ± 5.1) · 109/Land values of intact animals (660.0 ± 25.0) · 109/L. Similar changes occurred in terms of thrombocrit, average platelet volume and platelet distribution.
ISSN 1563-034Х |
Eurasian Journal of Ecology. №1 (58). 2019 |
89 |
еISSN 2617-7358 |
|
|
Pharmacological properties of endemic plants growing in the steppes of Kazakhstan
Figure 1 – Peripheral blood hemogram: total leukocyte indicators, · 109 / L (A),
absolute lymphocyte evidence, · 109 / L (B), absolute monocytice eosinophilic indicators, · 109 / L (C),
and absolute granulocytic indicators, · 109 / L (D). 1-data of the group with the introduction of the compound EES.SES/N, 2-data of the group with the introduction of the compound EES.SEB/N, 3-data of the group with the introduction
of the compound CIE.SV, 4-data of intact animals, 5-data of the control group with pantohematogen, 6-placebo group data with the introduction of nat. solution. The abscissa axis – the number of groups, the axis of ordinateblood indicates.
Figure 2 – Peripheral blood hemogram: total erythrocyte indicators, · 1012 / L (E) and hemoglobin index, g / L(F), total platelet indicators, · 109 / L. 1-data of the group with the introduction of the compound EES.SES / N, 2-data of the group
with the introduction of the compound EES.SEB/N, 3-data of the group with the introduction of the compound CIE.SV, 4-data of intact animals, 5-data of the control group with the introduction pantohematogen, 6-placebo group data with the introduction of nat. solution. The abscissa axis – the number of groups, the axis of ordinate – blood indices.
90 |
Хабаршы. Экология сериясы. №1 (58). 2019 |
