
Reference_01_08_2014_165529
.pdf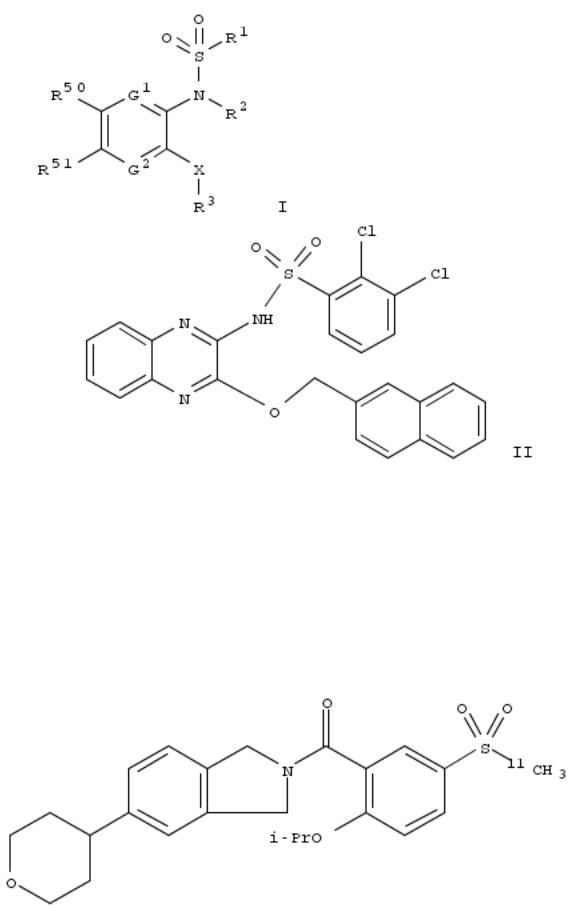
SciFinder® |
Page 91 |
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
122. Radiolabelled inhibitors of the glycine 1 transporter
By Alberati, Daniela; Borroni, Edilio Maurizio; Hartung, Thomas; Norcross, Roger; Pinard, Emmanuel
From U.S. Pat. Appl. Publ. (2010), US 20100111862 A1 20100506, Language: English, Database: CAPLUS
The present invention relates to novel radiolabeled inhibitors of formula I for the Glycine 1 transporter (GlyT1), useful for the labeling and diagnostic imaging of the glycine 1 transporter functionality.wherein R1 is isopropoxy or 2,2,2-trifluoro-1- methyl-ethoxy; andR2 is a radiolabeled group CH3, wherein the radionuclide is 3H or 11C. The radiolabeled compds. of formula I may be used as PET (Positron Emission Tomog.) radiotracer for the labeling and diagnostic mol. imaging of the glycine 1 transporter functionality.
~0 Citings

SciFinder® |
Page 92 |
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
123. First principles design of derivatizing agent for direct determination of enantiomeric purity of chiral alcohols and amines by NMR spectroscopy
By Orlov, Nikolay V.; Ananikov, Valentine P.
From Chemical Communications (Cambridge, United Kingdom) (2010), 46(18), 3212-3214. Language: English, Database: CAPLUS, DOI:10.1039/b923359h
77Se NMR offers superior sensing of chirality within the structure of the diastereomers (Δδ up to 6.1 ppm), compared to 13C (Δδ < 1 ppm) and 1H (Δδ < 0.2 ppm). The developed procedure is equally well suitable for detn. of the enantiomeric purity of chiral alcs. and amines as pure samples as well as reaction mixts. and crude products.
~8 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
124. Preparation of phthalazine compounds as p38 MAP kinase modulators
By Tasker, Andrew; Falsey, James Richard; Rzasa, Robert M.; Herberich, Bradley J.; Zhang, Dawei From PCT Int. Appl. (2010), WO 2010042649 A2 20100415, Language: English, Database: CAPLUS
The title compds. I [A4 = CR5 or N; L = NR4 or S; R1 = alkyl, alkoxy, NH(alkyl), etc.; R2 = H, F, Cl, Br, CF3, etc.; R3 = H, F, Cl, Br, CF3, etc.; R4 = H or alkyl; R5 = H, F, Br, Cl, I, etc.; m = 0-4], useful for the prophylaxis and treatment of protein kinase mediated diseases, including inflammation and related conditions, were prepd. and formulated. E.g., a multi-step synthesis of II, starting from 6-bromophthalazin-1-ol, was given. Exemplified compds. I were tested in various biol. tests (data provided for representative compds. I). The invention also comprises pharmaceutical compns. including one or more compds. I, uses of such compds. and compns. for treatment of p38 MAP kinase mediated diseases including rheumatoid arthritis, psoriasis, chronic obstructive pulmonary disease, ankylosing spondylitis, pain and other inflammatory disorders, as well as intermediates and processes useful for the prepn. I.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
125. Preparation of novel fused aminodihydrothiazine derivatives as β-secretase 1 (BACE 1) inhibitors
By Motoki, Takafumi; Takeda, Kunitoshi; Kita, Yoichi; Takaishi, Mamoru; Suzuki, Yuichi; Ishida, Tasuku From PCT Int. Appl. (2010), WO 2010038686 A1 20100408, Language: Japanese, Database: CAPLUS
SciFinder® |
Page 93 |
Fused aminodihydrothiazine compds. represented by general formula [I; ring A = (un)substituted C6-14 aryl, 5- or 6- membered heteroaryl, or 9- or 10-membered benzo-fused heterocyclic ring; L - a single bond, O, N-(un)substituted NHCO, NHCO-C1-6 alkyl, or NHSO2, each (un)substituted C1-6 alkylene, C2-6 alkenylene, or C2-6 alkynylene; ring B = each (un)substituted C3-8 cycloalkyl, C6-14 aryl, or 5- to 10-membered heterocycle ring; X = a single bond, (un)substituted C1-3 alkylene; Y = each (un)substituted C1-3 alkylene or C2-3 alkenylene; Z = O, S, S(O), S(O)2, (un)substituted NH; R1, R2 = H, each (un)substituted C1-6 alkyl, C1-6 alkylcarbonyl, C6-14 arylcarbonyl, C1-6 alkylsulfonyl, C6-14 arylsulfonyl, C3-8 carbocyclyl, or 5- to 10-membered heterocyclyl; R3-R6 = H, halo, HO, each (un)substituted C1-6 alkyl, C1-6 alkoxy, 3- to 10-membered carbocyclyl, or 5- to 10-membered heterocyclyl], pharmaceutically acceptable salts thereof, or solvates of the compds. or salts were prepd. These compds. have an amyloid β (Aβ) protein prodn.-inhibitory activity or BACE 1 inhibitory activity, and are thus useful as agents for treating neurodegenerative diseases caused by Aβ as typified by Alzheimer's disease and Down's syndrome. Thus, a suspension of 50 mg 5-cyanopyridine-2-carboxylic acid in 2 mL CH2Cl2 was treated with 140 μL oxalyl chloride under ice-cooling and treated with 4 mL THF to dissolve the compd. completely at the same temp. After evolution bubbles, the solvent was distd. away and the residue was dissolved in 5 mL THF to give a soln. of the acid chloride. The acid chloride soln. (788 μL) was added to a soln. of 14.5 mg (-)-[rel- (4aR,8aS)-8a-(5-amino-2-fluorophenyl)-4,4a,5,6,8,8a-hexahydro-7-oxa-3-thia-1-azanaphthalen-2-yl]carbamic acid tert-Bu ester in 5 mL THF under ice-cooling, followed by adding 500 μL pyridine at the same temp., and the resulting mixt. was stirred at room temp. for 2 h to give, after workup and removing the solvent, the amide. The amide was dissolved in 4 mL CH2Cl2, treated with 1 mL CF3CO2H, and stirred at room temp. for 3 h to give, after workup and NH-silica gel chromatog., 8.0 mg N-[3-(rel-(4aR,8aS)-2-amino-4,4a,5,6-tetrahydro-7-oxa-3-thia-1-azanaphthalen-8a-yl)-4- fluorophenyl]-5-cyanopyridine-2-carboxamide (I). I and N-[3-[rel-(4aR,6R,8aS)-2-amino-6-methoxymethyl-4,4a,5,6- tetrahydro-7-oxa-3-thia-1-azanaphthalen-8a-yl]-4-fluorophenyl]-5-difluoromethoxypyrazine-2-carboxamide (II) in vitro inhibited the prodn. Aα in rat brain nerve cells with IC50 of 0.0017 and 0.0004 μM, resp.

SciFinder® |
Page 94 |
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
126. Preparation of heterocyclylaminopyrazine derivatives for use as CHK-1 inhibitors
By Ninkovic, Sacha; Braganza, John Frederick; Collins, Michael Raymond; Kath, John Charles; Li, Hui; Richter, Daniel Tyler
From PCT Int. Appl. (2010), WO 2010016005 A1 20100211, Language: English, Database: CAPLUS

SciFinder® |
Page 95 |
Title compds. I [A = (un)substituted heteroaryl ring; R1 and R2 independently = H, F, Cl, CN, (un)substituted alkyl, etc.; R3 = represents 1 to 6 groups selected from F, CN, oxo, etc.; or two R3 attached to the same ring atom, together with the ring atom may form an (un)substituted ring selected from cycloalkyl, cycloalkenyl, or heterocyclyl; or two R3 attached to two adjacent ring atom, together with the ring atoms may form an (un)substituted fused ring selected from Ph, heteroaryl, cycloalkyl, etc.; or two R3 attached to two different ring atoms with at least one ring atom in between, may form an (un)substituted alkylene, heteroalkylene, or a diradical selected from O, NH, S, etc.; n = 0 to 2; with provisions], and their pharmaceutically acceptable salts, are prepd. and disclosed as CHK-1 inhibitors. Thus, e.g., II was prepd. by amination of 2,6-dichloropyrazine with 1,1-dimethylethyl ester (R)-3-amino-1-piperidinecarboxylic acid followed by heteroarylation with benzimidazole, and deprotection. I were evaluated in CHK-1 kinase inhibition assays, e.g., II demonstrated an Ki value of 0.0132 μM.
~9 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
127. Alkylthiazolyl carbamates, their preparation and use as fatty acid amido hydrolase (FAAH) inhibitors for treating FAAH-related pathologies
By Abouabdellah, Ahmed; Goerlitzer, Jochen; Hamley, Peter; Ravet, Antoine
From PCT Int. Appl. (2010), WO 2010010288 A2 20100128, Language: French, Database: CAPLUS
Title compds. I [R2 = H, F, OH, CN, CF3, alkyl, alkoxy, NH2 and derivs.; X = (CH2)n, Y = (CH2)m; n = 1-3; m = 1-2; A = a bong, alkylene; R1 = (un)substituted Ph, pyridazinyl, cinnolinyl, quinolinyl, etc.; R3 = H, F, alkyl, CF3; R4 = (un)substituted thiazolyl; their free bases and acid addn. salts; with the exclusion of specified compds.] were prepd. as fatty acid amido hydrolase (FAAH) inhibitors as well as endocannabinoids for FAAH-related pathologies. Thus, monoamination of 2,5- dibromopyridine with 2-(piperidin-4-yl)ethanol, cross-coupling of the bromide with 4-fluorophenylboronic acid, treatment with phthalimide, hydrazinolysis, and reaction of the primary amine with (4-nitrophenyl) thiazol-4-ylmethyl carbonate gave carbamate II. In a radioenzymic test, I displayed IC50 values ranging from 0.001 to 1 μM towards FAAH inhibition. I, at a dose of 1 to 30 mg/kg, reduced by 35 to 80% the abdominal stretches induced by i.p. administration of phenylbrnzoquinone, demonstrating analgesic activity.

SciFinder® |
Page 96 |
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
128. Synthesis and chiral recognition of novel amylose derivatives containing regioselectively benzoate and phenylcarbamate groups
By Shen, Jun; Ikai, Tomoyuki; Okamoto, Yoshio
From Journal of Chromatography A (2010), 1217(7), 1041-1047. Language: English, Database: CAPLUS, DOI:10.1016/j.chroma.2009.07.027
A new class of regioselectively substituted amylose derivs. bearing three different substituents at 2-, 3- and 6-positions, and two different substituents at 2-position and 3-, 6-positions were synthesized by a sequential process based on the esterification of 2-position of a glucose unit. Their chiral recognition abilities were evaluated as chiral stationary phases (CSPs) for high-performance liq. chromatog. (HPLC). Each deriv. had its own characteristic recognition ability depending on the arrangement of side chains at the three positions. Among the derivs., amylose 2-(4-t-butylbenzoate) and amylose 2-(4-chlorobenzoate) series exhibited high chiral recognition. Some racemates can be efficiently sepd. on these derivs. as well as on the amylose tris-3,5-dimethylphenylcarbamate, which is com. available as Chiralpak AD and one of the most powerful CSPs. The structures of the amylose derivs. were also investigated by CD spectroscopy.
~11 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
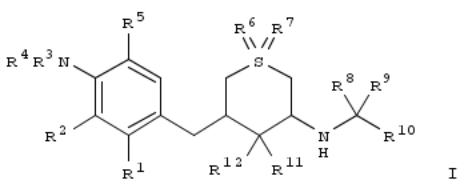
129. Preparation of aminobenzylthiopyranols as β-secretase (BACE) inhibitors.
By Briard, Emmanuelle; Lueoend, Rainer Martin; Machauer, Rainer; Moebitz, Henrik; Rogel, Olivier; Rondeau, JeanMichel; Rueeger, Heinrich; Tintelnot-Blomley, Marina; Veenstra, Siem Jacob
From PCT Int. Appl. (2010), WO 2010003976 A1 20100114, Language: English, Database: CAPLUS
SciFinder® |
Page 97 |
Title compds. [I; R1 = H, halo, alkyl; R2 = H, halo, alkyl, haloalkyl, alkoxy, haloalkoxy; R3 = H, R4 = H, alkoxyalkyl, alkylcarbonyloxyalkyl, aminoalkyl, CHO, etc.; or R3 = haloalkyl, hydroxyalkyl, alkoxyalkyl, CHO, alkylcarbonyl, etc., R4 = H, alkyl, alkoxyalkyl, CHO, alkylcarbonyloxyalkyl, alkylcarbonyl; R5 = H, halo, alkyl, haloalkyl, alkylaminoalkyl, alkenyl, haloalkoxy, aryl, aryloxy, heteroaryl, heteroaryloxy, etc.; R6, R7 = null, or R6 = O, R7 = null, or R6 = O, R7 = O, imino, alkylimino, benzylimino, formylimino, alkylcarbonylimino; R8, R9 = H, alkyl, haloalkyl, hydroxyalkyl, (substituted) cycloalkyl, etc.; R8R9 = cycloalkylidine, oxycycloalkylidene; R10 = (substituted) aryl, heteroaryl; R11 = H; R12 = OH; R11R12 = O], were prepd. Thus, (3S,4S,5R)-3-[4-amino-3-fluoro-5-[(R)-2,2,2-trifluoro-1-methoxymethylethoxy]benzyl]-5- (3-tert-butylbenzylamino)-1,1-dioxohexahydro-1λ6-thiopyran-4-ol [prepn. from (3R,4S,5S)-3-(3-tert-butylbenzylamino)-5- (3,5-difluoro-4-nitrobenzyl)-1,1-dioxohexahydro-1λ6-thiopyran-4-ol and (R)-1,1,1-trifluoro-3-methoxypropan-2-ol given] inhibited BACE with IC50 = 0.002 μM.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
130. Enantioseparation using amylose esters as chiral stationary phases for high-performance liquid chromatography
By Sugiura, Yuri; Yamamoto, Chiyo; Ikai, Tomoyuki; Kamigaito, Masami; Okamoto, Yoshio
From Polymer Journal (Tokyo, Japan) (2010), 42(1), 31-36. Language: English, Database: CAPLUS, DOI:10.1038/pj.2009.300
Novel amylose ester derivs. were synthesized and their chiral recognition abilities as chiral stationary phases for HPLC were evaluated. Compared with amylose benzoate derivs., cinnamate derivs. showed a higher chiral recognition ability and some racemates could be efficiently resolved. The recognition abilities of these derivs. varied significantly depending on the types and positions of substituents introduced into the Ph group. Among the prepd. derivs., the nonsubstituted cinnamate deriv. showed a relatively high chiral recognition, and interestingly, its ability was influenced by the prepn. conditions of packing materials using silica gel as a support.
~5 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
131. Asymmetric metallocene electrochromic compound and electrochromic apparatus comprising the compound
By Kim, Eun Gyeong; Yoo, Jeong Mok; Kim, Jeong Hwan
From Repub. Korean Kongkae Taeho Kongbo (2009), KR 2009130647 A 20091224, Language: Korean, Database: CAPLUS
The title compd. represented by the general formula I comprises an electrochromic part, an electroconductive part, and a connecting part used for connecting the electrochromic part and the electroconductive part and improving electrochromic characteristics, where R1 and R2 are independently C1-5 alkyl; R3 is selected from C1-20 alkyl with at least one hydrogen substituted by halogen atom, halo alkyl ether group, and halo alkyl ester group; R4 is elected from C1-5 alkyl, C2-5 alkenyl, C6-12 aryl, and C7-12 aralkyl; counter ion A- and B- are independently halogen ion, ClO4 -, BF4 -, PF6 -, AsF6 -, SbF6 -,
CHCOO-, CH3(C6H4)SO3 -, CF3SO3 -, (CF3SO2)2N-, (CF3CF2SO2)2N-, (CF3SO2)3C-, (CF3SO2)2N-, and Li(CF3SO2)2N-; m and n are independently integers of 0-4; Me is selected from Cr, Co, Fe, Mg, Ni, Os, Ru, and V. The compd. has the
advantages of high electrochromic response speed, good color contrast, and high stability.

SciFinder® |
Page 98 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
132. Symmetric metallocene electrochromic compound and electrochromic apparatus comprising the compound
By Kim, Eun Gyeong; Yoo, Jeong Mok; Kim, Jeong Hwan
From Repub. Korean Kongkae Taeho Kongbo (2009), KR 2009130646 A 20091224, Language: Korean, Database: CAPLUS
The title compd. represented by the general formula I comprises an electrochromic part, an electroconductive part, and a connecting part used for connecting the electrochromic part and the electroconductive part and improving electrochromic characteristics, where R1 and R2 are independently C1-5 alkyl; R3 is selected from C1-20 alkyl with at least one hydrogen substituted by halogen atom, halo alkyl ether group, and halo alkyl ester group; counter ion A- and B- are independently
halogen ion, ClO4 -, BF4 -, PF6 -, AsF6 -, SbF6 -, CHCOO-, CH3(C6H4)SO3 -, CF3SO3 -, (CF3SO2)2N-, (CF3CF2SO2)2N-, (CF3SO2)3C-, (CF3SO2)2N-, and Li(CF3SO2)2N-; m and n are independently integers of 0-4; Me is selected from Cr, Co,
Fe, Mg, Ni, Os, Ru, and V. The compd. has the advantages of high electrochromic response speed, high electrochromic persistence stability, and high electrochromic efficiency.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
133. Synthesis of 2-aryl-3,3,3-trifluoropropanoic acids using electrochemical carboxylation of (1-bromo-2,2,2- trifluoroethyl)arenes and its application to the synthesis of β,β,β-trifluorinated non-steroidal anti-inflammatory drugs
By Yamauchi, Yusuke; Hara, Shoji; Senboku, Hisanori
From Tetrahedron (2010), 66(2), 473-479. Language: English, Database: CAPLUS, DOI:10.1016/j.tet.2009.11.053

SciFinder® |
Page 99 |
(1-Bromo-2,2,2-trifluoroethyl)arenes were prepd. by reaction of aryl halides with a Weinreb amide to give the corresponding aryl trifluoromethyl ketones. Redn. of the ketones and treatment with tetrabromomethane or N- bromosuccinimide gave the desired bromo arenes. Electrochem. carboxylation of the (1-bromo-2,2,2- trifluoroethyl)arenes with a platinum cathode and a zinc anode resulted in an efficient fixation of carbon dioxide to give the corresponding 2-aryl-3,3,3-trifluoropropanoic acids in good yields. A probable reaction mechanism is discussed. This reaction was successfully applied to the synthesis of β,β,β-trifluorinated nonsteroidal anti-inflammatory drugs.
~11 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
134. Preparation of pyrazolopyrimidines as GPR119 receptor agonists
By Erickson, Shawn David; Gillespie, Paul; Guertin, Kevin Richard; Karnachi, Prabha Saba; Kim, Kyungjin; Ma, Chun; Mccomas, Warren William; Pietranico-Cole, Sherrie Lynn; Qi, Lida; Tilley, Jefferson Wright; et al
From U.S. Pat. Appl. Publ. (2009), US 20090286812 A1 20091119, Language: English, Database: CAPLUS
The title compds. I [R1 = OR2, NHR2, indolin-1-yl monosubstituted with SO2Me; R2 = (un)substituted aryl, heteroaryl, indole, etc.; R3 = cyclohexane substituted with oxadiazole substituted with lower alkyl, or piperidine substituted at N atom with R4; R4 = (un)substituted benzyl, heteroaryl, etc.; R5 = H, NH2, alkoxy, halo, alkyl; R6 = H, alkyl], useful for the treatment of metabolic diseases and disorders such as, for example, type II diabetes mellitus, were prepd. E.g., a multistep synthesis of II, starting from 1-tert-butoxycarbonyl-4-piperidone and hydrazine, was given. Exemplified compds. I were tested for their activity as GPR119 agonists in in vitro CAMP assay. For example, II showed EC50 of 0.83 μM. Pharmaceutical compn. contg. compds. I was disclosed.
~8 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
135. Preparation of amino acid-related imidazopyridine benzamide derivatives as anticancer agents
By Qian, Xiangping; McDonald, Andrew I.; Zhou, Han-Jie; Ashcraft, Luke W.; Yao, Bing; Jiang, Hong; Huang, Jennifer Kuo Chen; Wang, Jianchao; Morgans, David J., Jr.; Morgan, Bradley P.; et al
From U.S. (2009), US 7618981 B2 20091117, Language: English, Database: CAPLUS

SciFinder® |
Page 100 |
The invention relates to compds. I [R1 is H, cyano, nitro, or halo; R2 is alkoxy or CF3CHMeO; R3 is H, alkyl, alkoxy, halo, hydroxy, nitro, cyano, or amino; R4 is imidazo[1,2-a]pyridin-2-yl optionally substituted with one or two groups chosen from alkyl, halo, acyl, trifluoromethyl, aminomethyl, and hydroxyalkyl; R5 is H, halo, hydroxy, or alkyl; R6 is H or alkyl; R7 is hydroxyalkyl or a phosphate ester or acylaminoalkyl] and their pharmaceutically-acceptable salts, which are useful for the treatment of cancer or other proliferative disorders. One-hundred synthetic and four biol. examples are given. An example is compd. II, which was prepd. by a multistep sequence starting from Boc-protected 4-bromophenylalanine.
~5 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
136. Preparation of pyrrolo[3,4-c]pyrrole compounds as selective ligands for the neuronal nicotinic receptors and therapeutic uses for nervous system disorders
By Bunnelle, William H.; Shi, Lei; Scanio, Marc J.C.
From PCT Int. Appl. (2009), WO 2009137308 A1 20091112, Language: English, Database: CAPLUS
