
Met_OrgSynthesis
.pdfФедеральное агентство по образованию
Государственное образовательное учреждение высшего профессионального образования
«УФИМСКИЙ ГОСУДАРСТВЕННЫЙ НЕФТЯНОЙ ТЕХНИЧЕСКИЙ УНИВЕРСИТЕТ»
Кафедра иностранных языков
ORGANIC SYNTHESIS (PRACTICAL COURSE)
АНГЛИЙСКИЙ ЯЗЫК ДЛЯ СТУДЕНТОВ 2 КУРСА
ТЕХНОЛОГИЧЕСКОГО ФАКУЛЬТЕТА
УЧЕБНОЕ ПОСОБИЕ
УФА
2012
Данное учебное пособие предназначено для студентов 2 курса технологического факультета направлений 280700, 241000, 240100, 240000, 240700.
Пособие состоит из трех частей. Первая часть «Organic Chemistry» - содержит вводную информацию из курса органической химии, направленную на изучение и закрепление базовой лексики по профилю вуза и специальности. Вторая часть «Petrochemical Industry» содержит информацию об органическом синтезе углеводородов в нефтехимической промышленности. Тексты и задания к ним в каждой из этих частей расположены в порядке постепенного усложнения. Все тексты снабжены словарем с фонетической транскрипцией.
Третья часть содержит дополнительный материал, тексты которого могут быть использованы как для СРС, так и для аудиторных занятий.
Все тексты заимствованы из оригинальной английской и американской литературы.
Цель пособия – формирование умений и навыков чтения научнотехнических текстов по специальности с целью получения нужной информации, аннотирования и реферирования читаемой литературы, а также развития навыков устной речи на материале данной специальности.
Составители: В.А. Мельникова, старший преподаватель Д.Г. Халикова, преподаватель
Рецензенты: Л.О. Тимошенко, канд. филол. наук, доцент Кафедры иностранных языков гуманитарных факультетов БашГУ
Р. Р. Юсупова, канд. филол. наук, ст. преп. Кафедры делового иностранного языка и перевода Института права БашГУ
© Уфимский государственный нефтяной технический университет, 2012
1
CONTENTS
PART I. |
CHEMISTRY OF ORGANIC COMPOUNDS |
3 |
|
|
TEXT 1. |
Organic chemistry. Introduction |
3 |
|
TEXT 2. |
Organic chemistry. Historical highlights |
7 |
|
TEXT 3. |
Butlerov’s theory of organic compounds. Part I |
12 |
|
TEXT 4. |
Butlerov’s theory of organic compounds. Part II |
13 |
|
TEXT 5. |
Characteristics of organic substances |
20 |
|
TEXT 6. |
Hydrocarbons |
23 |
|
TEXT 7. |
Aliphatic and cyclic hydrocarbons |
27 |
|
TEXT 8. |
Polymers. Introduction into polymerization |
31 |
|
TEXT 9. |
Chain-growth and step-growth polymerization |
36 |
PART II. |
THE PETROLEUM-CHEMICAL INDUSTRY |
42 |
|
|
TEXT 1. |
General properties of petroleum |
42 |
|
TEXT 2. |
The petroleum-chemical industry |
46 |
|
TEXT 3. |
Synthetic rubbers |
51 |
|
TEXT 4. |
Polyethylene |
55 |
|
TEXT 5. |
Acetylene |
60 |
|
TEXT 6. |
Acetylene (II) (BASF) |
63 |
|
TEXT 7. |
Butylene and ethylene (Triolefin process) |
66 |
|
TEXT 8. |
Polyvinylchloride |
69 |
PART III. |
SUPPLEMENTARY READING |
70 |
|
|
TEXT 1. |
Methane |
70 |
|
TEXT 2. |
Ethylene |
73 |
|
TEXT 3. |
Acetylene |
77 |
|
TEXT 4. |
Benzene |
79 |
|
TEXT 5. |
Caprolactam (I) |
83 |
|
TEXT 6. |
Caprolactam (II) |
85 |
|
TEXT 7. |
Acrylates |
87 |
|
TEXT 8. |
Phthalic anhydride (I) |
89 |
|
TEXT 9. |
Phthalic anhydride (II) |
91 |
|
TEXT 10 |
Terephthalic acid |
93 |
|
TEXT 11. |
Sodium nitrilotriacetate (SNTA) |
94 |
|
TEXT 12. |
Styrene |
96 |
LITERATURE USED |
|
96 |
|
2
PART I. CHEMISTRY OF ORGANIC COMPOUNDS
TEXT 1. ORGANIC CHEMISTRY. INTRODUCTION
Organic chemistry is concerned with substances containing carbon. The number of known carbon compounds, or organic compounds, exceeds two millions, whereas inorganic substances number 50 thousands. Carbon may thus be singled out from all the chemical elements because of the remarkable diversity of its compounds.
Organic substances play an enormously important role in life. It is these substances that make up plant and animal organisms; they are constituents of our food (bread, meat, butter, and vegetables); they provide materials for the manufacture of clothing (fabrics, leather, etc.), form various types of fuel, and are used by us as drugs, dyes, and so on.
Organic substances have certain distinctive features. They are comparatively readily decomposed by heating, are mostly combustible, and their chemical reactions proceed more slowly as a rule than those of inorganic substances.
There is, however, no sharp dividing line between organic and inorganic substances. The carbon oxides, carbonic acid and its salts ought to be classified as organic substances because they contain carbon, but their properties are such that they are more closely related to inorganic substances.
Apart from carbon, the elements that organic substances contain most frequently are hydrogen, oxygen, and nitrogen; somewhat less frequent constituents are sulphur, phosphorus, the halogens, and certain metals.
Organic substances are easy to identify: when heated, they become charred or else burn with the formation of carbon dioxide.
The term “organic substances” arose early in the XIXth century, when many scientists believed that these substances were only produced by living organisms through the operation of a “vital force” and could not be produced artificially from inorganic substances. The doctrine concerning a “vital force”, known as vitalism, was unscientific, since it was founded on the belief in some supernatural power. By its contention that organic substances could not be created from inorganic matter, vitalism hampered scientific progress. Nevertheless, it could not, of course, halt the process of the accumulation of knowledge about nature.
In 1828 the German chemist F. Wöhler succeeded in preparing an organic substance (urea) from an inorganic for the first time. In 1854 the French chemist M. Berthelot prepared fats artificially. In 1861 the Russian scientist A.M. Butlerov carried out the first synthesis of a saccharoid substance. Syntheses of substances formerly produced by living organisms only began to follow one another in rapid succession.
Today chemists have not only synthesized many of the organic substances occurring in nature, but have prepared substances that do not occur in nature, such as plastics, various types of rubber, dyes, explosives, and medicines. Chemistry is now on the road to the artificial preparation of proteins, the most complicated substances of all, and beyond doubt this will be accomplished.
3

Tasks on the text |
|
|
|
|
|
|
|
|
|
|
|
||||
1. |
Memorize the following words and word combinations. |
|
|
|
|
||||||||||
1. |
substance [ |
|
] – |
|
12.combustible [ |
` |
] |
|
|
||||||
|
вещество |
|
|
|
– горючий, воспламеняемый |
|
|
|
|||||||
2. |
carbon [ |
|
|
] – углерод |
13.carbonic acid [ |
|
|
] – |
|||||||
3. |
to be concerned with – изучать, |
угольная кислота |
|
|
|
|
|||||||||
|
иметь дело с |
|
|
|
14.halogen [ |
|
|
] – галоген |
|
|
|||||
4. |
to exceed [ |
] – превышать |
15.to char [ |
] – обжигать, обугливать(ся) |
|||||||||||
5. |
to single out – выделять |
|
16.to hamper – препятствовать |
|
|
|
|||||||||
6. |
constituent [ |
|
|
] – |
17.to halt [h |
]– остановить |
|
|
|
||||||
|
компонент, составная часть |
18.urea [ |
] – мочевина |
|
|
|
|||||||||
7. |
fabric – ткань |
|
|
19.explosives [ |
|
|
] – взрывчатые |
||||||||
8. |
leather[` |
|
] – кожа |
|
вещества |
|
|
|
|
|
|
||||
9. |
drugs – лекарства |
|
|
20.saccharoid [ |
|
] – сахароид, |
|||||||||
10.dyes [ |
|
] – красители |
|
сахаровидный |
|
|
|
|
|
||||||
11.distinctive [ |
|
] – |
|
21.to accomplish [ |
] – совершать, |
||||||||||
|
отличительный, характерный |
выполнять; доводить до конца |
|
|
|||||||||||
2. |
Practise the pronunciation of the words given. Make sure you remember |
||||||||||||||
their meanings. |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
Organic [ |
|
], whereas [ |
|
], compound [ |
|
], diversity |
||||||||
[ |
|
], enormously [ |
|
], |
manufacture |
[ |
|
], |
various |
||||||
[ |
|
], |
feature [ |
], |
decomposed |
[ |
|
|
|
], proceed |
[ |
|
], |
||
however |
[ |
|
], |
oxide |
[ |
], |
|
frequently |
[ |
], |
hydrogen |
||||
[ |
|
|
], |
oxygen [ |
|
], nitrogen [ |
|
|
], sulphur |
[ |
], |
||||
phosphorus |
[ |
|
], charred [ |
], |
dioxide |
[ |
|
], vital |
[ |
], |
|||||
artificially [ |
|
|
], vitalism [ |
|
|
], unscientific [ |
|
|
], |
||||||
nevertheless [ |
|
], accumulation [ |
|
|
|
], chemist [ |
|
], |
|||||||
succeed |
[ |
|
], |
synthesis [ |
|
], |
syntheses |
[ |
], |
succession |
|||||
[ |
|
|
], synthesize [ |
|
], chemistry [ |
|
|
], protein [ |
|
|
]. |
||||
3.Read, translate and define what parts of speech the words, their derivatives and related words belong to. Consult the dictionary, write out the meanings that are new for you and memorize them.
Chemical – chemically – chemist – chemistry; constitute – constituent; variety
– various – variously – vary – varying; comparable – comparative – comparatively – compare – comparison; decompose – decomposed – decomposition; combustible – combustion – combustive; identical – identification – identify – identity; science – scientific – scientifically – scientist – unscientific; accumulate – accumulated – accumulation; succeed – succeedable – succeeder – succeeding – succeedingly.
4.Read paragraph one and compare organic compounds with inorganic ones in terms of their number.
5.Read paragraph two and say what it is about.
4
6.Find the emphatic sentence, translate it into Russian and comment on it.
7.Read paragraph three and answer the question: “What are distinctive features of organic substances?”
8.Find the sentence with the word “those”, translate it into Russian and state the meaning of this word.
9.Read the text up to the end and answer the following questions.
1)Is there a sharp dividing line between organic and inorganic substances?
2)What other elements apart from carbon do organic substances contain?
3)What do you know about vitalism?
4)What scientists succeeded in preparing organic substances?
5)Why is it so important to study organic chemistry?
10. Translate the following word combinations into English.
Количество известных соединений, играть очень важную роль, удивительное разнообразие, производство одежды, различные виды топлива, как правило, кроме углерода, с образованием двуокиси углерода, жизненная сила, накопление знаний, следовать друг за другом, в быстрой последовательности, вещества, встречающиеся в природе, искусственное создание белков, несомненно, дает нам возможность.
11.Translate the following sentences into Russian.
1)Органическая химия изучает вещества, содержащие углерод. Их количество превышает 2 млн. 2) Органические вещества сравнительно легко разлагаются при нагревании. 3) Эта доктрина основывалась на вере в некую сверхъестественную силу. 4) Витализм препятствовал научному прогрессу. 5) Немецкому химику Ф. Велеру удалось получить органическое вещество из неорганического. 6) Сегодня химики получили вещества, которые не встречаются в природе.
12.Find definition for each of the terms given.
a) |
carbon; |
e) |
dye; |
i) |
nitrogen; |
m) |
saccharoid; |
b) |
сarbonic acid; |
f) |
explosive; |
j) |
oxygen; |
n) |
sulphur (sulfur); |
c) |
carbon dioxide; |
g) |
halogen; |
k) |
phosphorus; |
o) |
synthesis; |
d) |
combustible; |
h) |
hydrogen; |
l) |
protein; |
p) |
urea. |
1.A nonmetallic element existing in crystalline forms: graphite, diamond, occurring in carbon dioxide, coal, oil, and all organic compounds. Symbol: C; atomic no.: 6; atomic wt.: 12.011; valency: 2, 3, or 4; relative density: 1.8-2.1 (amorphous), 1.9-2.3 (graphite), 3.15-3.53 (diamond); boiling pt.: 4827°C.
2.Any of a group of polysaccharides that remotely (отдаленно) resemble sugars, but are not sweet and are often insoluble.
3.A weak acid formed when carbon dioxide combines with water: obtained only in aqueous solutions, never in the pure state. Formula: H2CO3.
4.Any of the chemical elements fluorine (фтор), chlorine, bromine, iodine (йод), and astatine (астат). They are all monovalent and readily form negative ions.
5
5.A substance that decomposes rapidly under certain conditions with the production of gases, which expand by the heat of the reaction. The energy released is used in firearms, blasting, and rocket propulsion (движение).
6.An allotropic nonmetallic element, occurring free in volcanic regions and in combined state in gypsum, pyrite, and galena (галенит). It is used in the production of sulphuric acid, in the vulcanization of rubber. Symbol: S; atomic no.: 16; atomic wt.: 32.066; valency: 2, 4, or 6; relative density: 2.07 (rhombic),
1.957 (monoclinic); melting pt.: 115.22°C (rhombic), 119.0°C (monoclinic); boiling pt.: 444.674°C.
7.A white water-soluble crystalline compound with a saline (соленый) taste and often an odour of ammonia, produced by protein metabolism and excreted (выделяемый) in urine. A synthetic form is used as a fertilizer and in the manufacture of synthetic resins. Formula: CO(NH2)2 Also called: carbamide.
8.Any of a large group of nitrogenous compounds of high molecular weight that are essential constituents of all living organisms. They consist of one or more chains of amino acids linked by peptide bonds and are folded into a specific three-dimensional shape maintained by further chemical bonding.
9.An allotropic nonmetallic element occurring in phosphates and living matter. It is a toxic flammable phosphorescent (светящийся) white solid; the red form is less reactive and nontoxic: used in matches, pesticides, and alloys. Symbol: P; atomic no.: 15; atomic wt.: 30.973 762; valency: 3 or 5; relative density: 1.82
(white), 2.20 (red); melting pt.: 44.1°C (white); boiling pt.: 280°C (white).
10.A flammable colourless gas that is the lightest and most abundant element in the universe. It occurs mainly in water and in most organic compounds and is used in the production of ammonia and other chemicals, in the hydrogenation of fats and oils, and in welding. Symbol: H; atomic no.:1; atomic wt.: 1.00794; valency: 1; density: 0.08988 kg/m3; melting pt.: -259.34°C; boiling pt.: -252.87°C.
11.A colourless odourless highly reactive gaseous element: the most abundant element in the earth's crust (49.2 per cent). It is essential for aerobic respiration and almost all combustion. Symbol: O; atomic no.: 8; atomic wt.: 15.9994; valency: 2; density: 1.429 kg/m3; melting pt.: -218.79°C; boiling pt.: -182.97°C.
12.A colourless odourless relatively unreactive gaseous element (forms 78% of the air volume), occurs in many compounds, is an essential constituent of proteins and nucleic acids: used in the manufacture of ammonia and other chemicals and
as a refrigerant. Symbol: N; atomic no.: 7; atomic wt.: 14.00674; valency: 3 or 5; density: 1/2506 kg/m3; melting pt.: -210.00°C; boiling pt.: -195.8°C.
13.Capable of igniting and burning.
14.A liquid that contains a colouring material; can be used to stain fabrics,skins, etc.
15.A colourless odourless incombustible gas present in the atmosphere and formed during respiration, the decomposition, combustion of organic compounds, in the reaction of acids with carbonates: used in carbonated drinks, fire extinguishers, as dry ice for refrigeration. Formula: CO2. Also called: carbonic-acid gas.
16.The process of producing a compound by a chemical reaction or series of reactions, usually from simpler or commonly available starting materials.
6
TEXT 2. ORGANIC CHEMISTRY. HISTORICAL HIGHLIGHTS
At the beginning of the nineteenth century chemists generally thought that compounds from living organisms were too complicated in structure to be capable of artificial synthesis from non-living things, and that a 'vital force' or vitalism conferred the characteristics of living beings on this form of matter. They named these compounds 'organic', and preferred to direct their investigations toward inorganic materials that seemed more promising.
Organic chemistry received a boost when it was realized that these compounds could be treated in ways similar to inorganic compounds and could be created in the laboratory by means other than 'vital force'. Around 1816 Michel Chevreul started a study of soaps made from various fats and alkali. He separated the different acids that, in combination with the alkali, produced the soap. Since these were all individual compounds, he demonstrated that it was possible to make a chemical change in various fats (which traditionally come from organic sources), producing new compounds, without 'vital force'. In 1828 Friedrich Wöhler first manufactured the organic chemical urea (carbamide), a constituent of urine, from the inorganic ammonium cyanate NH4OCN, in what is now called the Wöhler synthesis.
Although Wöhler was cautious about claiming that he had thereby destroyed the theory of vital force, most have looked to this event as the turning point.
A great next step was when in 1856 William Henry Perkin, while trying to manufacture quinine, again accidentally came to manufacture the organic dye now called Perkin's mauve, which by generating a huge amount of money greatly increased interest in organic chemistry. Another step was the laboratory preparation of DDT by Othmer Zeidler in 1874, but the insecticide properties of this compound were not discovered until much later.
The crucial breakthrough for the theory of organic chemistry was the concept of chemical structure, developed independently and simultaneously by Friedrich August Kekule and Archibald Scott Couper in 1858. Both men suggested that tetravalent carbon atoms could link to each other to form a carbon lattice, and that the detailed patterns of atomic bonding could be discerned by skillful interpretations of appropriate chemical reactions.
The history of organic chemistry continues with the discovery of petroleum and its separation into fractions according to boiling ranges. The conversion of different compound types or individual compounds by various chemical processes created the petroleum chemistry leading to the birth of the petrochemical industry, which successfully manufactured artificial rubbers, the various organic adhesives, the property-modifying petroleum additives, and plastics.
The pharmaceutical industry began in the last decade of the 19th century when acetylsalicylic acid (commonly referred to as aspirin) manufacture was started in Germany by Bayer. The first time a drug was systematically improved was with arsphenamine (Salvarsan). Numerous derivatives of the dangerously toxic atoxyl were synthesized and tested by Paul Ehrlich and his group, and the compound with best effectiveness and toxicity characteristics was selected for production.
7

Biochemistry, the chemistry of living organisms, their structure and interactions in vitro and inside living systems, has only started in the 20th century, opening up a brand new chapter of organic chemistry with enormous scope.
Tasks on the text
1.Memorize the following words and word combinations.
1.complicated – сложный, запутанный
2.promising – перспективный
3.to confer [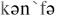 ] – давать, передавать
] – давать, передавать
4.boost – толчок, импульс
5.soap [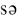 ] – мыло
] – мыло
6. |
alkali, -lis/ -lies [` |
] – щелочь |
|
7. |
carbamide [` |
|
] – карбамид, |
|
мочевина |
|
|
8. |
urine [` |
] – моча |
|
9. ammonium cyanate [ `
|
] – циановокислый аммоний, |
цианат аммония |
|
10.quinine [ |
] – хинин |
11.mauve [ |
] – розовато-лиловый |
12.Perkin’s mauve – анилиновый краситель (Перкина)
13.dichlorodiphenyltrichloroethane, DDT
– ДДТ, дихлордифенилтрихлорэтан
14.insecticide [ |
|
] – инсектицид, |
средство от насекомых |
|
|
15.crucial breakthrough [` |
: |
|
] – решающий шаг вперед, |
||
прорыв в науке |
|
|
16.simultaneously [, |
) |
] – |
вместе, одновременно; совместно
17.lattice [ |
] – решётка, сетка |
|
|
18.atomic bonding – (меж)атомная |
|
||
связь |
|
|
|
19.discern [d |
|
] – различать, |
|
распознавать, понять, отличать |
|
||
20.fraction – фракция, доля, частица |
|
||
21.boiling range – диапазон кипения |
|
||
22.petrochemical – нефтехимический |
|
||
23.adhesives [ |
` |
] – |
|
связывающие вещества |
|
||
24.modify [` |
|
] – изменять |
|
25.additives - присадки |
|
||
26.pharmaceutical [, |
] |
||
– фармацевтический |
|
||
27.acetylsalicylic [, |
,s |
|
|
cid – ацетилсалициловая кислота, |
|||
аспирин |
|
|
|
28.arsphenamine [ |
], |
|
|
salvarsan – сальварсан |
|
||
29.derivative [ |
|
] – |
|
производный, производное |
|
||
30.in vitro [ |
|
] – в пробирке; в |
|
искусственных условиях
31.brand new – принципиально новый
32.scope – границы, пределы, масштаб
2.Read, translate and define what parts of speech the words, their derivatives and related words belong to. Consult the dictionary, write out the meanings
that are new for you and memorize them.
Acid – acidify – acidize – acidly – acidness –acidulous; effective – effectively
– effectiveness; success – successful – succession – unsuccessful; syntheses – synthesis – synthesize –synthetic; toxic – toxicity – toxin – intoxication.
3.Read the title of the text and say what this text is about judging by its title. Look through the text to find the sentences that support your statement.
4.Read the text to decide what kind of information – explanatory, clarifying or additional – this text contains as compared to the previous one.
8
5.Rearrange the following sentences in the chronological sequence of events.
1)Michel Chevreul separated the different acids that, in combination with the alkali, produced the soap.
2)Another step was the laboratory preparation of DDT.
3)Chemists thought that compounds from living organisms were too complicated in structure to be capable of artificial synthesis from non-living things.
4)Friedrich Wöhler destroyed the theory of vital force.
5)Kekule and Couper developed the concept of chemical structure.
6)Wöhler first manufactured the organic chemical urea from inorganic ammonium cyanate.
7)William Henry Perkin manufactured the organic dye.
8)Biochemistry opened up a brand new chapter of organic chemistry.
9)The petroleum chemistry led to the birth of petrochemical industry.
10)They suggested that tetravalent carbon atoms could link to each other to form a carbon lattice.
11)In Germany acetylsalicylic acid manufacture was started.
12)The conversion of different compound types or individual compounds by various processes created the petroleum chemistry.
6.Answer the following questions:
1)Why did chemists prefer to direct the investigations toward inorganic materials?
2)When did organic chemistry receive a boost?
3)What did Michel Chevreul begin to study around 1816?
4)What event has been considered to be the turning point in organic chemistry?
5)How was the organic dye, now called the Perkin’s mauve manufactured?
6)What did it result in?
7)When were the insecticide properties of DDT discovered?
8)What was the crucial breakthrough for the theory of organic chemistry?
9)What can you say about a carbon lattice?
10)What are the products of petrochemical industry?
11)What did Paul Ehrlich and his group do?
12)What does biochemistry study?
7.Translate the following sentences into Russian.
1)Когда были получены некоторые составляющие и соединения живой материи из неорганических веществ, такие как щелочь и цианат аммония, теория о невозможности искусственного синтеза была разрушена. 2) Развитие органической химии получило толчок, когда лабораторным способом открыли некоторые свойства органических веществ. 3) Мишель Шеврёль получил мыло путем искусственного синтеза кислоты и щелочи. 4) Синтезом Велера было названо получение органической химической мочевины. 5) Анилиновый краситель Перкина был синтезирован им случайно, когда он пытался получить хинин. 6) Нефтехимическая промышленность занимается преобразованием различных углеродсодержащих соединений для получения искусственных резин, органических связывающих веществ, различных присадок, пластиков.
9
