
- •Water in the atmosphere
- •The names are: Mushroom, blind, blood, glazed rains. And still there is one
- •Distinctive properties of the water
- •Water melting and evaporation latent heat is rather high
- •Evaporation and condensation
- •Thermodynamics of the water phase transfer
- •Transfer from one phase to another goes at the constant temperature
- •The formula above can be transformed in the following way:
- •Magnus curve
- •Triple point
- •Other factors influencing the saturation water vapor pressure
- •When using original Thomson’s formula, it is worth knowing that the surface tension
- •In the real atmosphere water vapor condenses over so called condensation nuclei (ядрах
Water in the atmosphere
Water in the atmosphere makes all variety of the weather. An important element of the weather is cloudiness. There are many types of clouds. Some of them are beautiful, some gloom, some are terrible.
Of course, a fat lot they care what we think of them, but it is not so for us. The form, shape, and movement of clouds are indicators of the processes occurring in the atmosphere. It means that the clouds can serve as predictors of the weather. Therefore, the real meteorologists should be familiar with all aspects of the cloud “life”.
Precipitation, like cloudiness, are of different kind: rain, snow, drizzle, sleet etc. In turn, the rains can be divided into different forms: widespread, pouring, drizzling. There are some special form of rain. Every of them has its inherent name.
1
The names are: Mushroom, blind, blood, glazed rains. And still there is one particular one the people call “TSAREVNA’S TEARS”. That is the rain which can not be named by any other name but this.
The drops of this rain look larger than usual raindrops, They seem to fall slower from a small cloud. The Sun shinning from a side and reflecting from the drops makes them as if they are made with gold. Being transparent, they bear blue color from that part of the sky which is not covered with clouds.
Gold and blue combination makes them so beautiful that the name “ tsarevna’s tears” seems to be the most appropriate name for this rain.
2
Distinctive properties of the water
The water in the atmosphere is found in all three phases: vapor, liquid, and ice.
Special character of the water density variation.Other kindred |
||||||||||||||||||||
w T 00 C 1g / cm3 |
|
|
Density |
|
substances |
Water |
||||||||||||||
|
|
|
||||||||||||||||||
|
|
|
|
|
|
0.91g / cm3 |
|
|
|
|
|
|
|
|
||||||
ice |
T 0 |
0 |
C |
|
|
|
|
|
|
|
|
|
T°C |
|||||||
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0° |
4° |
|
|
||
Water heat capacity significantly changes when congealing to the |
||||||||||||||||||||
|
solid state. |
C |
w |
4187 J |
kg K |
C |
2114 |
J |
kg K |
That is |
||||||||||
|
|
|
ice |
|
|
|
||||||||||||||
|
not so for other kindred substances. |
|
|
|
|
|
|
|
|
|
||||||||||
Water freezing and boiling temperature are much higher than
that of other kindred chemical combinations.
3

Water melting and evaporation latent heat is rather high
L |
324 kJ |
kg |
L 2500 kJ |
kg |
m |
|
|
Water is a good solvent, it has chemical activity (iron rusts as contacting water)
Liquid water density varies with temperature a little. It can be
regarded as constant (1 g/cm³), as well as ice (0.91 g/cm³).
Water vapor heat capacity can be also regarded as non- depended on temperature variation.
C |
wv |
1386 J |
kg K |
C |
pw |
1846 J |
kg K |
|
|
|
|
C |
v |
718 J |
kg K |
C |
p |
1005 J |
kg K |
|
|
|
|
4
Evaporation and condensation
|
|
|
A number of molecules comes off, Noff |
|||||||
|
|
|
A number of molecules comes back, Nback |
|||||||
T T0 |
|
Noff |
Nback |
|
|
|
Evaporation |
T T |
||
|
|
|||||||||
|
Noff |
Nback |
|
|
|
Saturation state at |
||||
|
|
|
|
|
||||||
|
|
|
||||||||
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
0 |
|
|
At T1 T0 Noff |
Nback |
|
|
Condensation |
|
|||
|
|
|
|
|||||||
If the temperature in the closed space increases to become T2 T1, |
||||||||||
evaporation will begin again until |
Noff Nback |
|
||||||||
e E |
|
Evaporation goes on |
Noff Nback |
|
|
|||||
|
|
|
||||||||
e E |
|
Saturation has been reached |
Noff |
Nback |
|
|||||
|
|
|||||||||
e E |
|
Condensation takes place |
Noff Nback |
|
||||||
|
|
|||||||||
5
Thermodynamics of the water phase transfer
The following processes for the water phase transfer from state 1 to state 2 are possible
Water vapor |
Ice |
 Liquid water
Liquid water 
The first law of thermodynamics can be applied to describe the water
phase transfer processes
dq du edv
Let’s introduce the notion of thermodynamic potential Ф u ev T
denotes entropy (it is a term known from general physics) d dq |
||||
|
|
|
|
T |
|
dq Td du edv |
|
du edv Td 0 |
|
6

Transfer from one phase to another goes at the constant temperature
2
dq T 2 1 u2 u1 E v2 v1
1
T 2 T 1 u2 u1 Ev2 Ev1
|
u1 Ev1 T 1 |
|
u2 Ev2 T 2 |
|
|
|
|
|
|||||||||
|
|
Ф1 Ф2 |
|||||||||||||||
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ф1 |
|
|
|
|
|
Ф2 |
|
|
|
|
|
|
|||
|
Thermodynamic potential remains unchanged at the water phase |
||||||||||||||||
|
|
|
|
|
transfer from one state to another. |
|
|
|
|
||||||||
At temperature T+dT, Ф+dФ, and E+dE., and |
Ф1 dФ1 |
Ф2 dФ2 |
|||||||||||||||
|
|
|
Ф u ev T |
, |
dФ du Edv Td vdE dT |
||||||||||||
Recal ling that |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
dE |
|
|
|
|
|
dФ vdE dT, |
|
|
0 |
|||||||
|
|
|
|
|
|
|
|
||||||||||
|
2 |
1 |
v1dE 1dT v2dE 2dT |
||||||||||||||
|
dT |
|
v |
v |
|||||||||||||
|
|
|
|
2 1 dT v2 |
v1 dE |
||||||||||||
|
|
|
|
2 |
1 |
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7 |
|||
|
|
|
|
|
|
dq Td ; |
|
|
|
|
|
|
|
|
|
|
|
|
dq |
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
d T ; |
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
2 |
dq |
1 |
2 |
|
|
|
|
L1,2 |
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 dq |
|
|
|
|
Here L1,2 is specific latent heat |
|||||||||
|
|
2 |
1 |
|
1 T |
T |
T |
|
|||||||||||||||||||||||||
|
|
|
that is released as water |
||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
transfers from state 1 to state 2 |
|||||
|
|
dE |
|
|
|
L1,2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
Clausius – Clapeyron |
|
For practical |
||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
equation |
|
|
|
||||||||||||
|
|
dT |
|
T v2 |
v1 |
|
|
|
|
|
|
|
|
purposes the |
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
quantity 2,72 Тis |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
neglected because it |
|||||||||||||||||
|
For the process |
|
|
1. Liquid water |
|
|
|
|
|
2. Water vapor |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
is very small (T°C). |
|||||||||||||||||||||||||
|
|
|
|
|
L L 2,72 T 2500 2,72T kJ |
|
|||||||||||||||||||||||||||
|
|
L |
kg |
Therefore, the latent |
|||||||||||||||||||||||||||||
|
|
1,2 |
|
|
|
|
|
|
0 |
2500 kJ |
|
const |
|
|
heat is adopted as |
||||||||||||||||||
v2 v1 |
|
L L |
kg |
|
|
|
constant. |
|
|||||||||||||||||||||||||
|
|
|
|
|
L |
|
|
|
|
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
T 500 C 2,72 T 136 |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
dE |
|
|
|
|
|
|
Ev RwT |
dE |
|
|
|
|
LE |
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
L 2364 kJ kg |
||||||||||||||||||||||
dT |
|
T v2 |
|
|
|
|
RwT |
|
|
|
|
|
|
|
|||||||||||||||||||
|
|
|
|
|
|
|
dT |
|
|
|
|
RwT |
|
|
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
v E |
|
|
|
2 |
|
T 500 C 2,72 T 136 |
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
L 2636 kJ |
kg 8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The formula above can be transformed in the following way:
dE |
|
LE |
|
|
|
|
dE |
LdT |
||||
dT |
|
R T 2 |
|
|
|
|
E |
|
R T 2 |
|
||
|
|
|
|
|
|
|
||||||
|
|
w |
|
|
|
|
|
|
|
w |
||
|
|
|
|
|
|
|
T0 273.15K |
|||||
Adopting |
|
|
|
|
E0 6.1078hPa |
|||||||
|
|
|
|
|||||||||
|
|
|
|
|
|
|
R |
460 J |
kg K |
|||
|
|
|
|
|
|
|
w |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
Doing the same reasoning, we’ll obtain the similar formula for sublimation process.
1. Ice

 Water vapor
Water vapor
Much better results were obtained with empirically determined coefficients “a” and “b”
For water |
a=7.63 |
b=241.9 |
E |
dE |
|
|
L T dT |
|
|||||||||
E |
|
|
|
|
|
|
T T 2 |
|
||||||
|
E |
R |
|
|
||||||||||
0 |
|
|
|
|
|
w |
|
|
0 |
|
|
|||
ln |
E |
|
L |
|
1 |
|
|
1 |
|
|||||
|
|
|
|
|
|
|||||||||
|
R |
|
|
|
|
|
||||||||
|
E |
0 |
|
T |
|
T |
|
|||||||
|
|
|
|
w |
0 |
|
|
|
|
|||||
8.62T E E0 10273.15 T
9.76T Ei E0 10273.15 T
aT
E E0 10b T
For ice |
a=9.5 |
b=265.5 |
Magnus formula |
9
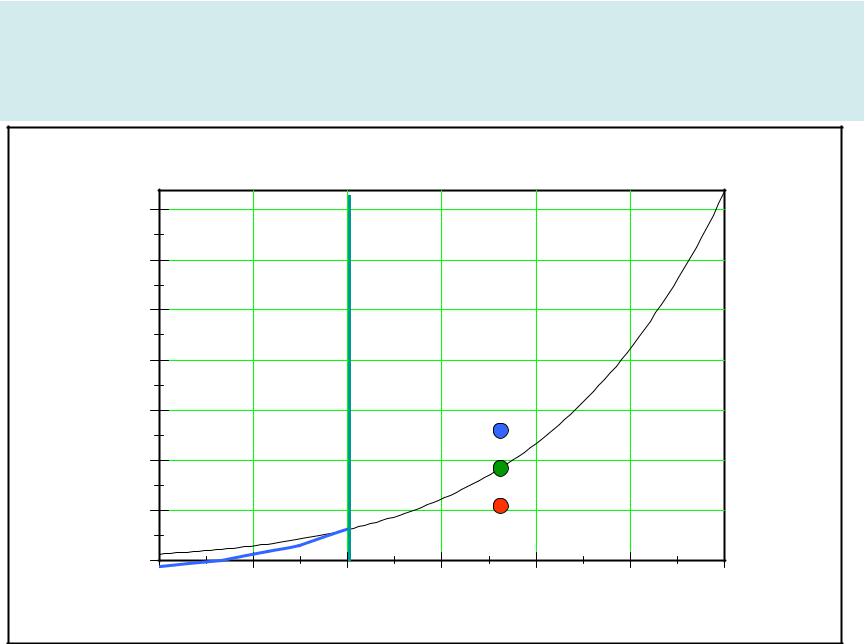
Magnus curve
|
70 |
|
|
|
|
|
|
|
|
|
|
|
|
|
60 |
|
|
|
|
|
|
|
|
|
|
|
|
|
50 |
|
|
|
|
|
|
|
|
|
|
|
|
hPa |
40 |
|
|
|
|
|
|
|
|
|
Water |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
Ice |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
30 |
|
|
|
|
|
|
|
|
|
B |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20 |
|
|
|
|
|
|
led |
|
C |
Water |
|
|
|
|
|
|
|
|
|
o |
|
|
|
|
|
|
|
|
|
|
|
|
o |
|
|
|
|
|
|
|
|
|
|
|
|
r |
c |
|
|
|
|
|
|
|
|
|
|
|
e |
|
|
|
|
|
|
|
|
|
|
|
|
p |
|
|
|
|
|
|
|
|
|
|
|
|
u |
|
|
|
|
|
e |
|
|
vapor |
|
|
|
|
S |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
dic |
|
|
|
|||
|
|
|
|
|
|
n |
|
|
|
A |
|
|
|
|
10 |
|
ater |
a |
|
|
|
|
|
|
|||
|
|
w |
|
|
|
|
|
|
|
|
|
|
|
|
0 |
|
|
|
|
|
|
|
|
|
|
|
T°C |
|
|
-20 |
|
-10 |
|
|
|
0 |
10 |
20 |
30 |
40 |
|
A-Under saturated air B-Super saturated air |
C-Saturated air |
10
