
Radiation Physics for Medical Physiscists - E.B. Podgorsak
.pdf
6 Interactions of Neutrons with Matter
Neutrons, by virtue of their neutrality, are indirectly ionizing radiation exhibiting a quasi-exponential penetration into an absorber and depositing energy in the absorber through a two-step process: (1) energy transfer to heavy charged particles and (2) energy deposition in the absorber through Coulomb interactions of the charged particles with atoms of the absorber. As they penetrate into matter, neutrons may undergo elastic and inelastic scattering as well as nuclear reactions, such as capture, spallation or fission. Two distinct categories of neutrons are of importance in medical physics: thermal neutrons used in boron-neutron capture therapy (BNCT) and fast neutrons used in external beam radiotherapy.
Several parameters used for describing neutron fields and neutron dose deposition in absorbers are defined and discussed in this chapter. Also discussed are several radiotherapy techniques based on neutron beams, machines for production of neutron beams in radiotherapy, and an e cient source of neutrons for brachytherapy, the californium-252.
170 6 Interactions of Neutrons with Matter
6.1 General Aspects of Neutron Interactions with Absorbers
Neutrons, similarly to photons, may penetrate an absorber without interacting or they may undergo various interactions with the absorber. In contrast to photons, however, neutrons interact mostly with the nuclei of the absorber and have only weak interactions with orbital electrons of the absorber.
Neutron beams, similarly to photon beams, belong to the category of indirectly ionizing radiation beams, both types transferring energy to absorbing medium through an intermediate step in which energy is transferred to a charged particle (protons and heavier nuclei in the case of neutrons; electrons and positrons in the case of photons).
The secondary heavy charged particles released in a medium traversed by neutrons have a very short range in the medium ensuring charged particle equilibrium. Since no bremsstrahlung x rays are generated by the charged particles, the absorbed dose for neutron beams is equal to kerma at any point in the neutron field.
In terms of their kinetic energy EK, neutrons are classified into several categories:
1.Ultracold neutrons with EK < 2 × 10−7 eV
2.Very cold neutrons with 2 × 10−7eV ≤ EK ≤ 5 × 10−5 eV
3.Cold neutrons with 5 × 10−5 eV ≤ EK ≤ 0.025 eV
4.Thermal neutrons with EK ≈ 0.025 eV,
5.Epithermal neutrons with 1 eV < EK < 1 keV,
6.Intermediate neutrons with 1 keV < EK < 0.1 MeV,
7.Fast neutrons with EK > 0.1 MeV.
Note that the velocity of an ultracold neutron with kinetic energy of 2 × 10−7 eV is 6 m/s (υ/c ≈ 2 × 10−8); of a thermal neutron with kinetic energy of 0.025 eV it is 2200 m/s (υ/c ≈ 7 × 10−6); and of a fast neutron with kinetic energy of 2.5 MeV it is 1.4 × 107 m/s (υ/c ≈ 0.05).
There are five principal processes by which neutrons interact with the nuclei of the absorber:
1.Elastic scattering,
2.Inelastic scattering,
3.Neutron capture,
4.Spallation,
5.Fission.
The probability (cross section) for these di erent types of interactions varies with the kinetic energy of the neutron and with the properties of the absorber.

6.2 Neutron Interactions with Nuclei of the Absorber |
171 |
6.2 Neutron Interactions with Nuclei of the Absorber
6.2.1 Elastic Scattering
In elastic scattering a neutron collides with a nucleus of mass M that recoils with an angle φ with respect to the neutron initial direction of motion, as shown schematically in Fig. 4.2 and discussed in Sect. 4.3. Kinetic energy and momentum are conserved in the interaction.
For a neutron with mass mn and initial kinetic energy EKi, the kinetic energy ∆EK transferred to the nucleus is in general given as (see Sect. 4.3.1)
∆EK = EKi |
4mnM |
cos2 |
φ . |
(6.1) |
|
(mn + M )2 |
|||||
|
|
|
|
The maximum possible energy transfer (∆EK)max is attained in a head-on collision for which φ = 180◦ (see Sect. 4.3.1)
4mnM |
|
(∆EK)max = EKi (mn + M )2 . |
(6.2) |
The average kinetic energy ∆EK transferred to the recoil nucleus is
|
K = |
1 |
EKi |
4mnM |
= 2EKi |
mnM |
|
(6.3) |
|
∆E |
. |
||||||||
2 |
(mn + M )2 |
(mn + M )2 |
|||||||
|
|
|
|
|
|
The kinetic energy of the scattered neutron, EKf , in a head-on collision is equal to
mn − M 2
EKf = EKi − (∆EK)max = EKi , (6.4)
mn + M
while EKf , the average energy attained by the scattered neutron, is
m2 + M 2
EKf = EKi − ∆EK = EKi n 2 . (6.5)
(mn + M )
Thus, for example, if the target nucleus is hydrogen (nucleus is a proton with mass mp), then M = mp ≈ mn and the neutron will transfer on the average one half of its initial kinetic energy to the proton [see (6.5)]. The maximum energy transferred to the proton equals to the initial neutron energy EKi [see (6.2)]. The recoil proton will then travel a short distance through the absorbing medium and rapidly transfer its kinetic energy to the medium through Coulomb interactions with the nuclei and orbital electrons of the medium.
The transfer of the neutron’s energy to the absorbing medium is much less e cient when mn M ; the larger is M , the less e cient is the energy transfer, as evident from (6.2).
172 6 Interactions of Neutrons with Matter
6.2.2 Inelastic Scattering
In inelastic scattering the neutron n is first captured by the nucleus and then re-emitted with a lower energy and in a direction that is di erent from the incident neutron direction. The nucleus is left in an excited state and will de-excite by emitting high energy gamma rays. This process is illustrated by the following relationship:
where |
n + ZAX → ZA+1Y → ZAX + n ZAX → ZAX + γ , |
(6.6) |
|
|
|
ZAX |
is the target nucleus, |
|
A+1Y |
is an unstable compound nucleus, |
|
Z |
|
|
ZAX |
is an excited target nucleus. |
|
6.2.3 Neutron Capture
Neutron capture is a term used to describe a nuclear reaction in which a thermal neutron bombards a nucleus leading to the emission of a proton or gamma ray. Two of these interactions are of particular importance in tissue: 14N(n,p)14C and 1H(n, γ)2H and one interaction, 113Cd(n,γ)114Cd, is of importance in shielding against thermal neutrons.
A cadmium filter with a thickness of 1 mm absorbs essentially all incident thermal neutrons with energies below 0.5 eV, but readily transmits neutrons with energies exceeding 0.5 eV. The cross section for neutron capture plotted against neutron kinetic energy exhibits a broad resonance with a peak at 0.178 eV. At the resonance peak energy the cross section for neutron capture by natural cadmium (12% abundance of cadmium-113) is 7800 b, while pure cadmium-113 has a cross section of 64 × 103 b.
Often neutron bombardment of a stable target is carried out in a nuclear reactor with the intent of producing a radioactive isotope for industrial or medical purposes. When the main interest in the reaction is the end product, the reaction is termed neutron activation. Of interest in medical physics is the neutron activation process in general and in particular when it is used for production of cobalt-60 sources for radiotherapy, iridium-192 sources for brachytherapy, and molybdenum radionuclides for nuclear medicine diagnostic procedures. The neutron activation process is discussed in greater detail in Sect. 8.4.
6.2.4 Spallation
Spallation occurs when a fast neutron n penetrates the nucleus and adds sufficient energy to the nucleus so that it disintegrates into many small residual components such as α particles and protons p. An example of spallation is as follows:
8160 + n → 3α + 2p + 3n . |
(6.7) |
6.2 Neutron Interactions with Nuclei of the Absorber |
173 |
Most of the energy released from the spallation process is carried away by the heavier fragments that deposit their energy in the absorber locally. On the other hand, neutrons and de-excitation gamma rays produced in spallation carry their energy to a remote location.
6.2.5 Fission Induced by Neutron Bombardment
Fission is a particular type of neutron interaction produced by the bombardment of certain very high atomic number nuclei by thermal neutrons. The residual particles are nuclei of lower atomic number and usually more than one fast neutron is produced by the reaction. The discovery of fission is attributed to Otto Hahn, Fritz Strassman, Lise Meitner and Otto Frisch in 1939.
Materials that can undergo the fission reaction are called fissile or fissionable materials. The most important fissionable materials are:
–Uranium-235 (0.7% of naturally occurring uranium)
–Plutonium-239 produced from uranium-238
–Uranium-233 produced from thorium-232
•Uranium-238 and thorium-232 are called fertile nuclides; they do not undergo fission themselves; however, they transform into fissionable nuclides upon bombardment with neutrons in a nuclear reactor.
•As fissionable nuclides undergo the fission process, lighter, generally radioactive, nuclides called fission fragments are formed. Fission fragments combined with the nuclides subsequently formed through radioactive decay of fission fragments are called fission products.
A general equation for fission of uranium-235 is as follows: |
|
92235U + n → 92236U → ab X + dc Y + f n , |
(6.8) |
where the nucleus 23592 U has been penetrated by a thermal neutron n to produce a compound nucleus 23692 U. The compound nucleus 23692 U is unstable and divides by the fission process into two generally unstable nuclei of smaller atomic number and atomic mass number such that a + c = 92 and b + d + f = 236, with f the number of fast neutrons produced by the fission process.
Fission reactions always result in the release of a large amount of energy. On the average, the energy released is about 200 MeV per fission of 23592 U and the number of new neutrons produced is, on the average, 2.5 per fission.
The new neutrons can be used to produce fission in other uranium-235 nuclei leading to an exponential increase in the number of neutrons and resulting in a nuclear chain reaction. The kinetic energy acquired by fission fragments is converted into heat that can be used in nuclear reactors in a controlled fashion for peaceful purposes in electric power generation. Unfortunately, uncontrolled chain reactions can be used for destructive purposes
174 6 Interactions of Neutrons with Matter
either directly in atomic bombs or indirectly as detonators of fusion-based hydrogen bombs.
In a nuclear reactor the nuclear chain reactions are controlled in such a way that, following each fission, only one new neutron is used for continuing the chain reaction. The first nuclear reactor was constructed in 1942 in Chicago under the scientific leadership of Enrico Fermi. Since then several hundred nuclear reactors have been constructed around the world, mainly for electric power generation but also for research purposes and for production of radionuclides used in industry and medicine.
The principal component of any reactor is the core that contains the fissionable fuel, most commonly uranium oxyde with uranium-235 enriched to 2–4% in contrast to its natural abundance of 0.7%.
Fission occurs in the nuclear fuel and the fission energy in the form of kinetic energy of fission fragments and new neutrons is rapidly converted into heat. A coolant (usually water) is used to maintain a stable temperature in the reactor core. The coolant exits the core either as steam or as hot pressurized water, subsequently used to drive turbines connected to electric power generators. The neutron fluence rate in the reactor core is controlled by movable control rods that are made of material with high cross section for absorption of neutrons, such as cadmium or boron compounds.
The fission e ciency is the highest for thermal neutrons, but the new neutrons are produced with relatively large kinetic energies. Moderators are used to slow down the new neutrons through elastic scattering events between neutrons and nuclei of the moderator. Water serves as moderator material in most reactors; however, some reactors may use the so-called heavy water (D2O, where D stands for deuterium), graphite or beryllium for the purposes of moderation. Heavy water has a smaller probability for neutron absorption through the (n, γ) reaction than water. Graphite also does not absorb many neutrons and scatters neutrons well. Beryllium is an excellent solid moderator with its low neutron absorption cross section and a high neutron scattering cross section.
6.3 Neutron Kerma
Neutron fields are usually described in terms of fluence ϕ(EK) rather than energy fluence ψ(EK) as is usually the case with photon fields. For a monoenergetic neutron beam of fluence ϕ in cm−2 undergoing a specific type of interaction i with a particular atom at a point in medium, the kerma Ki in a small mass m is expressed as
Ki = ϕ σi |
N |
( |
|
K)i |
(6.9) |
|
∆E |
||||||
m |
||||||
|
|
|
|
|
where

6.4 Neutron Kerma Factor |
175 |
ϕis the neutron fluence in cm−2
σi |
is the cross section for the particular interaction i, |
Nis the number of target atoms in mass m with N/m = NA/A,
(∆EK)i is the mean energy transferred from neutrons to charged particles through the particular interaction i.
The product σiN/m summed over all possible neutron interactions is the mass attenuation coe cient µ/ρ for neutrons in the absorbing medium.
Following the convention used for photon beams, we define the mass energy transfer coe cient µtr/ρ for neutrons as follows:
µ tr |
|
µ |
|
|
|
|
|
= |
|
∆EK |
, |
(6.10) |
|||
ρ |
|
|
|||||
|
ρ EK |
|
|||||
where ∆EK/EK is the fraction of the neutron energy transferred to charged particles.
The total kerma K accounting for all possible interactions is
K = ϕ σi |
N |
|
|
K = ϕ |
µ |
|
K = ϕ |
µtr |
EK , |
(6.11) |
|||
∆E |
∆E |
||||||||||||
m |
|
ρ |
|
|
|||||||||
|
|
|
|
|
|
|
ρ |
|
|||||
where EK is the kinetic energy of the monoenergetic neutron beam.
6.4 Neutron Kerma Factor
The product (µtr/ρ)EK, defined as the neutron kerma factor Fn with units of J · cm2/g, is tabulated for neutrons instead of the mass energy transfer coe cient. Figure 6.1 provides the neutron kerma factor Fn against neutron kinetic energy for various materials of interest in medical physics (hydrogen, water, tissue, carbon, oxygen, and nitrogen).
For monoenergetic neutrons we get the following expression for the neutron kerma K:
K = ϕ (Fn)EK,Z , |
(6.12) |
where
ϕis the fluence of monoenergetic neutrons of kinetic energy EK,
(Fn)EK,Z is the neutron kerma factor Fn in J · cm2/g for neutrons of kinetic energy EK in the irradiated absorber with atomic number Z.
For neutron beams characterized with an energy spectrum ϕ (EK) of particle fluence, kerma is expressed as follows:
|
(EK)max |
|
|
K = |
|
ϕ (EK)(Fn)EK,ZdEK , |
(6.13) |
|
0 |
|
|
where (EK)max is the maximum neutron kinetic energy in the continuous neutron spectrum with the di erential fluence distribution ϕ (EK).
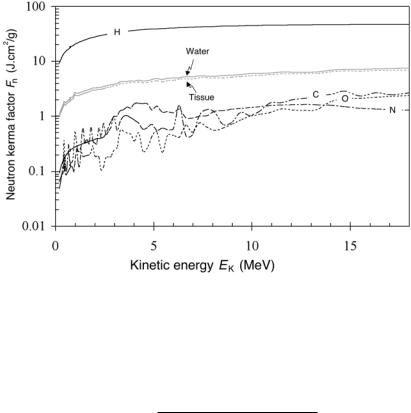
176 6 Interactions of Neutrons with Matter
Fig. 6.1. Neuton kerma factor Fn against neutron kinetic energy EK for various materials of interest in medical physics. Data were obtained from the NIST
An average value for the neutron kerma factor Fn for the spectrum of neutrons ϕ (EK) is given as
(EK)max
|
|
|
K |
0 |
ϕ (EK)(Fn)EK,ZdEK |
|
|
|
|
|
|
|
|
|
|||
(Fn)ϕ (EK),Z = |
|
= |
|
|
. |
(6.14) |
||
ϕ |
EKmax |
|||||||
ϕ (EK) dEK
0
6.5 Neutron Dose Deposition in Tissue
By virtue of their neutrality, neutrons, similarly to photons, deposit dose in tissue through a two-step process:
1.Energy transfer to heavy charged particles, such as protons and heavier nuclei in tissue.
2.Energy deposition in tissue by heavy charged particles through Coulomb interactions of the charged particles with atoms of tissue.
Similarly to photons, the nature of neutron interactions with tissue depends on the kinetic energy of neutrons; however, the options available for neutron interactions are not as varied as those for photons (see Chap. 7). For neutrons there are only two kinetic energy ranges to consider:
1.Thermal neutron energy of the order of 0.025 eV.
2.Epithermal, intermediate and fast neutrons with kinetic energy >0.025 eV.

6.5 Neutron Dose Deposition in Tissue |
177 |
6.5.1 Thermal Neutron Interactions in Tissue
Thermal neutrons undergo two possible interactions with nuclei of tissue:
1.Neutron capture by nitrogen-14 (147 N) nucleus that produces carbon-14 (146 C) and a proton. The cross section for the 147 N(n,p)146 C reaction is σN−14 = 1.84 b/atom.
2.Neutron capture by hydrogen-1 (11H) nucleus that produces a deuterium nucleus and a γ photon. The cross section for reaction 11H(n,γ)21H is σH−1 = 0.33 b/atom.
According to the ICRU and the ICRP the human tissue composition in percent by mass is: 10% for hydrogen-1 and 3% for nitrogen-14. The data for oxygen-16 and carbon-12, the other two abundant constituents of tissue, are 75% and 12%, respectively.
The kerma deposited in muscle tissue per unit neutron fluence ϕ is from (6.9) given as follows:
K |
= σ |
Nt |
|
|
|
(6.15) |
|
∆EK , |
|||||||
ϕ |
m |
||||||
|
|
|
|
|
|||
where
σis the thermal neutron cross section for the specific nuclear reaction,
∆EK is the average energy transfer in the nuclear reaction,
(Nt/m) is the number of specific nuclei, such as nitrogen-14 or hydrogen-1, per unit mass of tissue.
Thermal Neutron Capture in Nitrogen-14 in Tissue
The kinetic energy released by thermal neutron capture in nitrogen-14 is determined by calculating the change in total nuclear binding energy between the nitrogen-14 nucleus (EB = 104.66 MeV) and the carbon-14 nucleus (EB = 105.29 MeV). Since the total binding energy of carbon-14 exceeds that of nitrogen-14 by 0.63 MeV, we note that the energy released to charged particles in thermal neutron capture by the nitrogen-14 nucleus is 0.63 MeV. This energy is shared as kinetic energy between the proton and the carbon14 nucleus in the inverse proportion of their masses, since both nuclei carry away the same momenta, but in opposite directions. Thus, the proton receives a kinetic energy of 0.58 MeV; the carbon-14 atom a kinetic energy of 0.05 MeV.
The number of nitrogen-14 atoms per gram of tissue, (Nt/m)N−14, is determined as follows:
1.1 gram-atom of N-14 contains NA atoms of N-14.
2.1 g of N-14 contains (NA/A) atoms of N-14, where A = 14.01 g/gramatom.
178 6 Interactions of Neutrons with Matter
3.1 g of tissue contains 0.03 g of N-14 atoms, i.e., 0.03 × (NA/A) atoms of N-14, therefore (Nt/m)N−14 = 1.3 × 1021 atom/g.
The kerma K per unit thermal neutron fluence ϕ for the 147 N(n,p)146 C reaction is thus equal to
K |
= σ |
|
Nt |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
∆EK |
|
|
|
|
|
|
|
|||||||||||
ϕ |
|
|
m |
|
|
|
|
|
|
|
|||||||||
|
|
|
N−14 |
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
= 1.84 |
× |
10−28 |
m−2 |
|
|
1.3 |
× |
1021 |
atom |
× |
0.63 MeV |
|||||||
|
|
|
g |
||||||||||||||||
|
|
|
|
|
atom × |
|
|
|
|||||||||||
|
= 2.4 |
× |
10−17 Gy |
· |
m−2 |
/neutron. |
|
(6.16) |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Thermal Neutron Capture in Hydrogen-1 in Tissue
Despite a lower cross section for capture in hydrogen compared to nitrogen, thermal neutrons have a much larger probability for being captured by hydrogen than by nitrogen in tissue because in the number of atoms per gram of tissue (concentration) hydrogen surpasses nitrogen with a ratio of 45 to 1.
In the 11H(n, γ) 21H reaction a γ photon is produced and the binding energy di erence between a proton EB = 0 and deuteron (EB = 2.22 MeV) is 2.22 MeV. Neglecting the recoil energy of the deuteron, we assume that the γ photon receives the complete available energy of 2.22 MeV, i.e., Eγ = 2.22 MeV.
The number of hydrogen-1 atoms per gram of tissue, (Nt/m)H−1 is determined as follows:
1.1 gram-atom of H-1 contains NA atoms of H-1.
2.1 g of H-1 contains (NA/A) atoms of H-1.
3.1 g of tissue contains 0.1 g of H-1 atoms, i.e., 0.1 × (NA/A) atoms of H-1, therefore (Nt/m)H−1 = 6 × 1022 atom/g ≈ 45 × (Nt/m)N−14.
The energy transfer to γ photons per unit thermal neutron fluence ϕ and per unit mass of tissue m for the 11H(n,γ)21H nuclear reaction is given as follows, again using (6.9):
Eγ |
|
= σH−1 |
|
Nt |
|
∆Eγ |
|
|
|
|
|
|
||
ϕm |
|
m |
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
H−1 |
|
|
|
|
|
|
||
|
|
= 0.33 |
× |
10−28 |
m−2 |
|
6 |
× |
1022 |
atom |
× |
2.22 MeV |
||
|
|
|
g |
|||||||||||
|
|
|
|
|
atom × |
|
|
|
||||||
|
|
= 7 × 10−16 J · kg−1 · m−2/neutron . |
|
(6.17) |
||||||||||
The result of (6.17) represents the energy per unit neutron fluence and per unit mass of tissue that is transferred to γ photons. The amount of this energy that actually contributes to the kerma in tissue depends on the fraction of this energy that is transferred from the γ photons to electrons in tissue. This fraction depends on the size of the tissue mass: for a small size mass most of the γ photons may escape; for a large mass all photons might be absorbed.
