
Micro-Nano Technology for Genomics and Proteomics BioMEMs - Ozkan
.pdfPEPTIDE ARRAYS IN PROTEOMICS AND DRUG DISCOVERY |
173 |
either the initiation of peptide synthesis or the immobilization of peptides. It was often demonstrated that the surface-bound peptides’ accessibility to the proteins or enzymes used in screening is a critical factor. Insertion of a spacer between the peptide and the surface is an effective way to circumvent this drawback (Figure 7.11B). Generally, all linker molecules introduced to transform a given surface function into a functional group suitable for amino acid or peptide attachment can be considered as spacers. Such spacers can improve the efficiency of peptide/ligand interaction on surfaces as demonstrated with FLAG epitope peptides recognized by the monoclonal anti-FLAG M2 antibody [581]. The signal increased with the length of spacer introduced between the epitope and the surface. Additionally, for protein tyrosine kinase p60c-src it was demonstrated that only incorporation of the long and hydrophilic 1-amino-4,7,10-trioxa-13-tridecanamine succinimic acid building block spacer allowed effective phosphorylation of the glass surface-bound peptides [143]. Moreover, insertion of hydrophilic dextran structures between the surface and the presented peptides (Figures 7.12 and 7.13) was described as necessary for efficient enzyme substrate interaction [337].
The loading of glass surfaces is too low for several biochemical reactions (Tables 7.1, 7.2). Several different approaches for generating dendrimeric spacers/linkers are used to increase the density of immobilized peptides (Figure 7.11C, 7.12–7.16). Alternatively, the effective surface per area can be increased by the use of porous silicon [306, 347, 462]. Examples for one-step generation of dendrimeric structures (Figure 7.11C) are represented by poly-lysine coated glass slides (Table 7.2) or aziridine polymerization onto aminopropylsilylated glass surfaces (Figure 7.16) [85, 257, 388]. An interesting approach for increasing the density of reactive functions is surface modification by adsorption of structured α-helical peptides [332] or proteins (bovine serum albumin, BSA; [338]) yielding amino modified polymers. The BSA amino functions were transformed into active esters by treatment with N,N-disuccinimidyl carbonate (Figure 7.15).
More sophisticated procedures for preparing multiple linker structures by multistep functionalization of glass surfaces have been reported (Figures 7.14, 7.17) [31, 33, 197, 242, 258, 302, 410]). Alternatively, surface loading could be improved by coatings employing three-dimensional layers such as hydrogels (Rubina et al., 2003), semi-wet gels [258], agarose films [5], acrylamide gel pads [367, 430, 565] or gelatine pads [136].
7.2.2. Generation of Micro-Structured Surfaces
Micro-structured or micro-patterned surfaces represent an alternative way to achieve spatially addressed deposition of molecules. This could be useful for generating microreactors [604] or improving array regularity. Two major principles are used for micropatterning: contact printing techniques (see Section 7.2.4.1.) and photolithografic technologies. The use of micro-contact printing for micro-patterning [36, 107, 225, 226, 240, 283, 284, 349, 507] has been reviewed extensively [60, 365, 366, 512, 601, 602] and is therefore not discussed in detail. Dip-Pen nanolithography/scanning probe lithography [51, 67, 249, 256, 302, 385, 519, 577, 580, 598] and microfluidic channel networks [83, 103, 105, 398] were used for nanoand micro-patterned immobilization of biomolecules such
174 |
ULRICH REINEKE, JENS SCHNEIDER-MERGENER AND MIKE SCHUTKOWSKI |
i
O
O
OH
O
O
HN
O |
O |
ii |
|
H2N |
O |
O |
N |
HO |
H |
iii
O
OH
O
OH
O
OH
OO
O |
OH |
O |
|
|
|
||
OH |
|
OH |
|
O |
O |
O |
|
|
|
HOH
N O
O N
H
O
O |
iv |
OH
O
OH
O
OH
OO
O |
OH |
O |
|
|
|
||
NH2 HN |
|
OH |
|
O |
O |
O |
HOH
N O
O N
H
NH2 O
FIGURE 7.12. Transformation of porous polypropylene into hydrophilic matrix suitable for enzyme profiling [337]. i = oxidation using Cr2O3/H2SO4/H2O, 54:54:80 (wt,wt,wt), ii = 5% oxalylchloride in CH2Cl2, 2 h, room temperature followed by treatment with 10% 2,2’-(ethylenedioxy)diethylamine in CH2Cl2, iii = carboxymethylated dextran, N-hydroxysuccinimide, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride in water, 12 h, room temperature, iv = carbonyl diimidazole, acetonitrile, 30 min, 0.3 M diaminopropane in acetonitrile, 1 h.

TABLE 7.2. Selected suppliers of modified surfaces
surface function |
world wide web |
dimension [mm] |
material |
|
amino |
www.aims-scientific-products.de |
80 |
× 120 |
cellulose |
amino |
|
100 |
× 150 |
cellulose |
amino |
|
235 |
× 250 |
cellulose |
streptavidin |
|
100 |
× 150 |
cellulose |
amino |
|
240 |
× 240 |
polypropylene |
amino |
|
80 |
× 120 |
polypropylene |
amino |
|
240 |
× 240 |
polypropylene |
amino |
|
80 |
× 120 |
polypropylene |
bromide |
|
100 |
× 150 |
cellulose |
carboxyl |
|
100 |
× 150 |
cellulose |
Fmoc-ß-Alanine |
|
100 |
× 150 |
cellulose |
Fmoc-Proline |
|
100 |
× 150 |
cellulose |
mercapto |
|
100 |
× 150 |
cellulose |
hydroxyl |
|
240 |
× 240 |
polypropylene |
hydroxyl |
|
80 |
× 120 |
polypropylene |
hydroxyl |
|
240 |
× 240 |
polypropylene |
hydroxyl |
|
80 |
× 120 |
polypropylene |
epoxy |
www.quantifoil.com |
75 |
× 25 |
glass |
aldehyde |
|
75 |
× 25 |
glass |
epoxy |
www.noabdiagnostics.com |
75 |
× 25 |
glass |
aldehyde |
|
75 |
× 25 |
glass |
NHS ester |
|
75 |
× 25 |
glass |
aldehyde |
www.aat-array.com |
75 |
× 25 |
glass |
amino |
www.sigmaaldrich.com |
75.5 |
× 25 |
glass |
poly-L-lysine |
|
75.5 |
× 25.5 |
glass |
carboxyl |
www.nuncbrand.com |
76 |
× 25 |
heat stable polymer |
amino |
|
76 |
× 25 |
glass |
epoxy |
|
76 |
× 25 |
glass |
aldehyde |
|
76 |
× 25 |
glass |
amino |
www.eriesci.com |
n.a. |
|
glass |
poly-lysine |
|
n.a. |
|
glass |
epoxy |
|
n.a. |
|
glass |
loading
400 nmol/ cm2
400 nmol/ cm2
400 nmol/ cm2
2 nmol/ cm2
80 nmol/ cm2
80 nmol/ cm2
600 nmol/ cm2
600 nmol/ cm2
800 nmol/ cm2
500 nmol/ cm2
800 nmol/ cm2
600 nmol/ cm2
600 nmol/ cm2
150 nmol/ cm2
150 nmol/ cm2
1500 nmol/ cm2
1500 nmol/ cm2 n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
n.a.
(cont.)
DISCOVERY DRUG AND PROTEOMICS IN ARRAYS PEPTIDE
175

TABLE 7.2. Continued
surface function |
world wide web |
|
dimension [mm] |
material |
loading |
|
|
|
|
|
|
amino |
www.arrayit.com |
76 |
× 25 |
glass |
8 pmol/cm2 |
amino |
|
76 |
× 25 |
glass |
8 pmol/cm2 |
aldehyde |
|
76 |
× 25 |
glass |
8 pmol/cm2 |
poly-L-Lysine |
|
76 |
× 25 |
glass |
n.a. |
acrylic |
|
76 |
× 25 |
glass |
8 pmol/cm2 |
carboxyl |
|
76 |
× 25 |
glass |
8 pmol/cm2 |
cyanato |
|
76 |
× 25 |
glass |
8 pmol/cm2 |
epoxy |
|
76 |
× 25 |
glass |
8 pmol/cm2 |
mercapto |
|
76 |
× 25 |
glass |
8 pmol/cm2 |
amino |
www.xenopore.com |
75 |
× 25 or 22 × 40 |
glass |
16 pmol/cm2 |
aldehyde |
|
75 |
× 25 or 22 × 40 |
glass |
16 pmol/cm2 |
epoxy |
|
75 |
× 25 or 22 × 40 |
glass |
16 pmol/cm2 |
maleimide |
|
75 |
× 25 or 22 × 40 |
glass |
16 pmol/cm2 |
nitrilotriacetic acid |
|
75 |
× 25 or 22 × 40 |
glass |
n.a. |
streptavidin |
|
75 |
× 25 or 22 × 40 |
glass |
n.a. |
biotin |
|
75 |
× 25 or 22 × 40 |
glass |
n.a. |
mercapto |
|
75 |
× 25 or 22 × 40 |
glass |
16 pmol/cm2 |
amino |
www.corning.com/lifesciences |
75.5 × 25.3 |
glass |
n.a. |
|
substituted antraquinones |
www.exiqon.com |
75 |
× 25 |
injection molded polymer |
n.a. |
amino |
www.greinerbioone.com |
75 |
× 25 |
glass |
n.a. |
aldehyde |
|
|
|
glass |
n.a. |
streptavidin |
|
|
× 25 or 24 × 60 |
glass |
n.a. |
epoxy |
www.asperbio.com |
75 |
glass |
n.a. |
|
amino |
|
75 |
× 25 or 24 × 60 |
glass |
n.a. |
isothiocyanate |
|
75 |
× 25 or 24 × 60 |
glass |
n.a. |
epoxy |
www.genescan.com |
75.7 × 25.4 |
glass |
16 pmol/mm2 |
|
amino |
www.perkinelmer.com/ |
75 |
× 25 |
glass coated with modified acrylamide |
n.a. |
|
areas/proteomics/chem2.asp |
active surface: 12 × 40 or |
polymer |
|
|
|
|
12 |
× 12 (two pad) |
|
|
176
SCHUTKOWSKI MIKE AND MERGENER-SCHNEIDER JENS REINEKE, ULRICH
epoxy |
www.mwgbiotech.com |
75 |
× 25 |
glass |
n.a. |
mercapto |
www.ApogentDiscoveries.com |
75 |
× 25 |
glass |
n.a. |
animo |
www.csem.ch |
75 |
× 25 |
glass |
n.a. |
carboxyl |
|
22 |
× 20 available for print |
metal oxides |
4.2 pmol/mm2 |
maleimide |
|
|
|
metal sulfides |
3.4 pmol/mm2 |
mercapto |
|
|
|
silicon |
|
|
|
|
|
silicon nitride |
|
|
|
75.5 × 25 |
plastics |
|
|
amino |
www.us.schott.com |
glass |
n.a. |
||
isothiocyanate |
www.picorapid.de |
76 |
× 26 |
glass |
n.a. |
amino-reactive |
www.ucb-group.com |
75 |
× 25 |
glass |
n.a. |
|
|
variable size |
cellophane |
n.a. |
|
|
|
variable size |
polypropylene |
n.a. |
|
amino |
biolink@bellatlantic.net |
120 × 80 |
highly crosslinked, polyaminated, |
15 nmol/mm2 |
|
|
|
|
× 25 (slide) |
polyurea coated polypropylene fleece |
|
amino |
www.pall.com |
75 |
positive charged nylon membrane bound |
n.a. |
|
|
|
60 |
× 22 (membrane) |
to glass |
|
aldehyde |
www.pall.com |
Ultrabind US450 membrane roll |
modified polyethersulfone |
n.a. |
|
|
|
3000 × 320 |
|
|
|
amine reactive |
www.accelr8.com |
75 |
× 25 |
glass or silicon |
n.a. |
thiol-reactive |
|
|
|
|
n.a. |
biotin |
|
|
× 25 |
|
n.a. |
NHS ester |
www1.amershambiosciences.com |
75 |
glass |
50 fmol/mm2 |
|
|
|
|
|
|
|
n.a. not available
DISCOVERY DRUG AND PROTEOMICS IN ARRAYS PEPTIDE
177

178 |
ULRICH REINEKE, JENS SCHNEIDER-MERGENER AND MIKE SCHUTKOWSKI |
i 
 X Y
X Y

R HN
CF3
ii
|
|
|
|
X Y |
|
|
S |
HN |
|
|
O |
HN |
|
CF3 |
|
OH |
|
|
|
|
|
|
|
|
OH |
O |
O |
|
|
|
OH |
O |
|
|
|
|
|
|
|
|
|
OH |
O |
|
|
|
|
|
|
|
|
NH |
OH |
|
|
S |
OH |
O |
|
|
|
OH |
|
|
|
|
NH |
O |
|
|
|
|
||
|
|
|
|
OH |
|
|
|
OH |
O |
|
|
|
OH |
|
|
|
|
|
CF3
N
N
FIGURE 7.13. Photobonding to and functionalization of surfaces such as glass, polystyrene, silicon nitride or polyurethane membranes. i = photochemical coupling of N-substituted m-3-(trifluoromethyl)diaziridin-3- yl-anilines, R = 4-maleimidobutyryl residue [91, 442]; ii = Optodex treatment [71].
as proteins, but no applications for the creation of peptide arrays have been described so far.
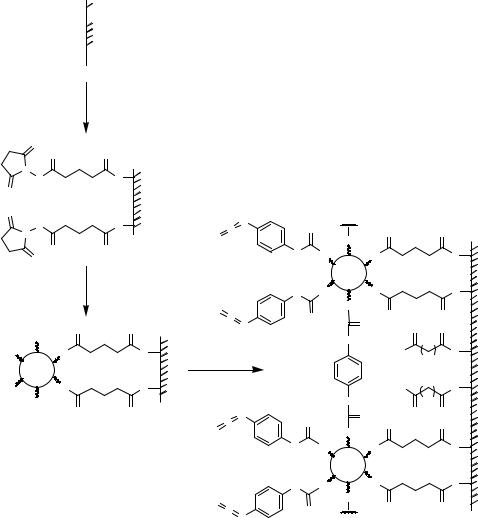
Surfaces modified by amino groups protected by photosensitive protection groups can be used to generate microstructures simply by irradiation through a lithographic mask [480, 605]. A very similar approach [613] starts from the derivatization of glass surfaces with a photolabile self-assembled monolayer (Figure 7.18). Irradiation led to release of hydrophobic moieties yielding hydrophilic, carboxy modified areas (B) within a hydrophobic (A) environment.
Alternatively, photoresist coatings can be used in combination with photolithographic masks to create micro-patterns. Following irradiation, areas protected by photoresist coatings will not undergo modification upon treatment with tridecafluoro- 1,1,2,2-tetrahydro)trichlorosilane in anhydrous toluene (Figure 7.19). After removal of the
PEPTIDE ARRAYS IN PROTEOMICS AND DRUG DISCOVERY |
179 |
H2N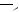

H2N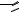
i
O
OO
N |
N |
|
O |
||
O |
H |
|
|
||
O |
H |
|
O |
||
N |
||
N |
|
OO
O
ii
OO
|
NH2 |
|
H2N |
HN |
N |
|
PAMAM |
H |
|
|
|
|
HN |
H |
H2N |
N |
|
|
NH2 |
|
OO
N |
S |
|
|
|
|
C |
|
O |
O |
|
|
S |
|
NH |
|
||
|
|
|
|
||
|
|
|
|
|
|
N |
NH |
HN |
|
|
N |
H |
|
|
|
|
H |
|
PAMAM |
|
|
|
|
H |
|
|
|
|
H |
NH |
HN |
|
|
N |
|
N |
|
|
|||
S |
|
NH |
O |
O |
|
S |
|
|
|||
C |
S |
|
|||
N |
|
O |
O |
|
|
|
|
|
|
||
|
|
NH |
|
|
|
|
|
|
HO |
3 |
N |
iii |
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
HO |
|
H |
|
|
|
3 |
N |
|
|
|
NH |
|
|
|
|
|
O |
O |
|
|
N |
|
S |
|
||
S |
O |
O |
|
||
C |
NH |
|
|||
S |
|
|
|
|
|
N |
NH |
HN |
|
|
N |
H |
PAMAM |
|
|
H |
|
|
|
|
|
||
H |
|
|
|
|
H |
NH |
HN |
|
|
N |
|
N |
|
|
|||
|
|
|
|
||
S |
|
NH |
O |
O |
|
|
|
|
|||
C |
S |
|
|
||
|
|
|
|
||
N |
|
|
|
|
|
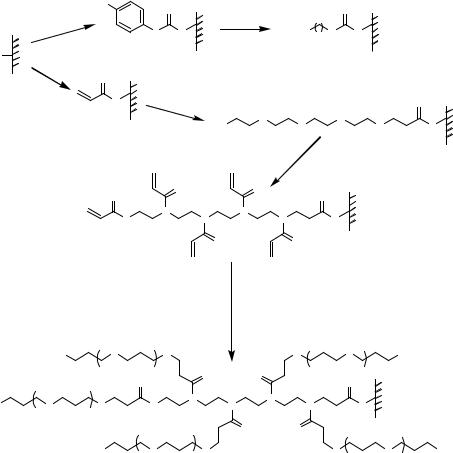
FIGURE 7.14. Glass surface modification/activation via starbust dendrimer coating [33]. i = treatment of aminosilylated glass with homobifunctional disuccinimidylglutarate in CH2Cl2 containing 1% diisopropylethylamine, 2 h; ii = 10% PANAM starbust monomer, 12 h; iii = activation and intermolecular crosslinking using homobifunctional 1,4-phenylenediisothiocyanate in CH2Cl2 containing 1% pyridine, 2 h.
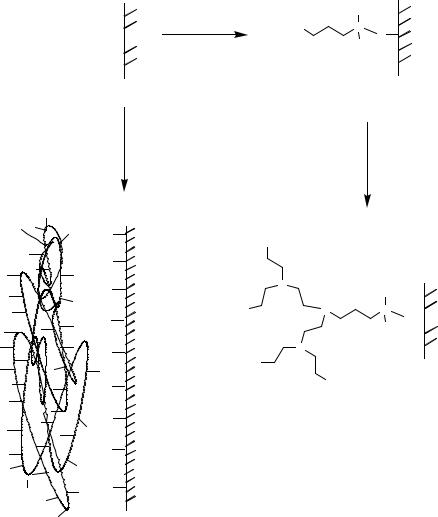
protecting layer the newly exposed glass surfaces can be transformed into hydrophilic areas by aminopropylsilylation and acylation. Butler and coworkers produced so-called surface tension arrays [70] for the on-chip synthesis of oligonucleotides using a similar principle (Figure 7.20). The resulting hydrophilic areas (B), surrounded by hydrophobic areas (A) with a very low surface tension, represent micro-reactors holding the reaction solvent in area B due to differences in the wetting characteristics. The perfluorosilane modified areas A have wetting properties similar to Teflon. Alternatively, Brennan used a laser for

180 |
ULRICH REINEKE, JENS SCHNEIDER-MERGENER AND MIKE SCHUTKOWSKI |
H2N
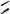
 i
i
OO
NN
 H
H 
O |
|
|
|
ii |
|
NH2 |
|
|
H2N |
|
O |
|
|
|
BSA |
N |
N |
|
H |
H |
H2N
NH2
i
O
O |
O |
|
N |
|
|
|
|
|
|
N |
O |
NH |
O |
|
|
|
|||
O |
|
|
|
O |
HN |
|
|
|
|
|
|
|
|
|
O |
|
BSA |
N |
N |
|
|
|
H |
H |
NHN
O O O NH
N
O O
FIGURE 7.15. Multistep functionalization of glass surfaces [338]. i = N,N-disuccinimidyl carbonate, diisopropylamine, dimethylformamide, 3 h, room temperature; ii = PBS pH 7.5, 1% bovine serum albumin (BSA), 12 h, room temperature.
PEPTIDE ARRAYS IN PROTEOMICS AND DRUG DISCOVERY
ii
HO 
i
|
NH2 |
|
|
|
|
H2N |
NH2 HO |
|
|
|
|
|
||
H2N |
HO |
|
||
|
H2N |
|
||
H2N |
NH2 |
|
||
|
|
|||
H2N |
|
HO |
|
|
|
NH2 |
|
||
H N |
|
H2N |
||
|
|
|||
2 |
|
NH HO |
|
|
H2N |
H2N |
|
||
2 |
|
|||
|
|
|
||
H2N |
|
NH2 HO |
|
|
H2N |
|
|
H N |
|
H2N |
H N |
NH |
2 |
|
2 |
||||
H2N |
2 |
|
||
H2N |
HO |
|
||
H2N |
|
|
||
H2N |
|
|
||
|
HO |
|
||
|
H2N |
|
||
H2N |
NH2 |
|
||
H2N |
|
|||
H2N |
H2N |
HO |
|
|
|
|
|
||
H N |
HN |
NH2 |
|
|
2 |
|
|
||
|
NH |
NH HO |
|
|
|
2 |
2 |
|
|
|
H2N |
|
|
|
|
H2N |
|
|
|
181
|
CH3 |
H2N |
Si |
|
O |
|
CH3 |
iii
NH2
N
CH3
N Si
O 
CH3
N
NH2
FIGURE 7.16. Aziridine-polymerization [85, 257, 388] and poly-lysine coating. i = the glass surface is coated by covering it with poly-L-lysine solution and drying the film; the polymer film is bound to the surface by electrostatic interactions between silyl-OH functions and positive charged amino groups in the polymer; ii = ethoxydimethylaminopropylsilane/toluene, iii = aziridine / methylene chloride / acetic acid.
ablation of fluorosiloxanes (Figure 7.21) to create micro-patterned surfaces starting from a uniformly hydrophobic modified glass slide [61]. Furthermore, photolithographic techniques have been reported for the micro-patterning of films ([188, 268, 381, 606]; reviewed in [186, 305]). Micro-mirror mediated patterning of biomolecules has been reported [303, 304, 510].
182 |
|
|
ULRICH REINEKE, JENS SCHNEIDER-MERGENER AND MIKE SCHUTKOWSKI |
|||||
|
|
|
O2N |
O |
|
O |
|
|
|
|
|
|
|
|
|
||
|
|
i |
O |
N |
ii |
nN |
|
|
|
|
H2N |
N |
|
||||
|
|
|
|
|||||
|
|
|
|
H |
|
H |
H |
|
H2N |
|
|
|
|
|
|
|
|
|
iii |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
iv |
H |
H |
|
O |
|
|
|
H |
|
|
|||
|
|
|
|
|
N |
N |
|
|
|
|
|
|
H2N |
N |
|
N |
N |
|
|
|
|
|
H |
|
H |
H |
|
|
|
|
|
v |
|
|
|
|
|
|
O |
O |
O |
O |
|
|
|
|
|
|
|
|
|
||
|
|
|
N |
N |
N |
N |
|
|
|
|
|
N |
N |
|
|
||
|
|
|
H |
|
|
H |
|
|
|
|
|
|
O |
O |
|
|
|
|
|
|
vi |
|
|
|
|
H |
H |
|
|
H2N |
O |
N |
N |
O |
NH2 |
|
|
2 |
|
|
2 |
OO
|
|
|
O |
|
|
O |
|
H N |
O |
N |
N |
N |
N |
N |
|
N |
N |
|
|||||
2 |
|
2 H |
H |
|
|
H |
|
|
|
|
|
O |
O |
|
|
|
|
H2N |
O |
2 N |
N |
O 2 |
NH2 |
|
|
|
|
H |
H |
|
|
FIGURE 7.17. Multistep functionalization of aminoalkylsilylated glass surfaces or plasma-aminated polypropylene surfaces [31]. i = 4-nitrophenylchloroformiate, diisopropylamine, CH2Cl2, 2 h; ii = diamine, dimethylformamide, 12 h; iii = acryloylchloride, diisopropylamine, dimethylformamide, 24 h, iv = tetraethylenepentamine, dimethylformamide, 12 h; v = acryloylchloride, diisopropylamine, dimethylformamide, 24 h; vi = 1,4-bis(3-aminopropoxy)-butane, dimethylformamide, 12 h.
7.2.3. Peptide Array Preparation
Peptides are usually synthesized step by step using appropriate side chain (permanent protecting group) and N-terminal protected (temporary protecting group) amino acid derivatives (Figure 7.22). Generally, fluorenyl-oxy-carbonyl- (Fmoc), tert-butyl-oxy-carbonyl- (Boc) and nitroveratryl-oxy-carbonyl (Nvoc) moieties are used as temporary protecting groups (PG) while preparing peptide arrays. Subsequent to amino acylation of surfacebound amino functions, the PG of the surface-bound amino acid derivative is removed by base (Fmoc), acid (Boc) or light (Nvoc) treatment. Repeated amino acylation and deprotection reactions yield a surface-bound target peptide in the side chain protected state. Final
