
Micro-Nano Technology for Genomics and Proteomics BioMEMs - Ozkan
.pdf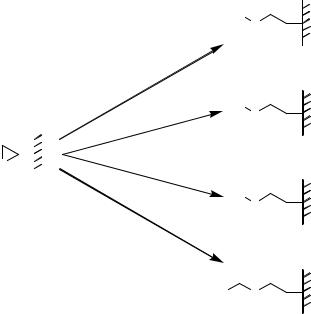
PEPTIDE ARRAYS IN PROTEOMICS AND DRUG DISCOVERY |
193 |
|
|
|
R |
||||
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
H |
|
|
|
|
|
|
|
OH |
||
|
|
R-NH2 |
|
|
|
|
|
|
|
|
R |
||||
|
|
R-OH |
|
|
O |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|||
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
O |
R-SH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
||||
|
|
R-COOH |
|
|
S |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
|
|
|
O |
||||
|
|
|
|
|
|
|
|
|
|
|
R O |
|
|
||
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
||
FIGURE 7.28. Chemistry of epoxy-modified surfaces. Versatility of epoxy functions demonstrated by reaction with primary amines, alcohols, mercaptanes and carboxylic acids yielding N-alkyl-aminoalcohols, monoalkylated diols, S-alkyl-mercaptoalcohols and monoacyl-diols, respectively.
should also be an attractive method for peptide immobilization. Compared with standard chemistry this method led to an approximately 35-fold higher biological signal subsequent to spotting a 10 µM sample solution.
The epoxy function represents the most versatile moiety for non-specific immobilization because it can react with nearly all possible side chain functionalities of the deposited peptide (Figure 7.28). Examples of non-specific immobilization chemistries are given in Figures 7.1i, 7.1ii, 7.1iii, 7.1v, 7.6i, 7.7v, 7.8iii, 7.9, 7.14, 7.15, 7.17i, 7.17iii, 7.17v, 7.29ii, 7.29iv, and 7.30i.
Another possibility for non-selective immobilization is the use of surface modifications that can be activated by light generating reactive surface functionalities. Treatment of silicone wavers with 2-nitro-5-[11-(trimethoxysilyl)undecyl]oxybenzyl methoxy poly(ethylene glycol) propanoate yielded surfaces covered with masked aldehyde functionalities [384]. Irradiation with UV-light (330 nm) generates the aldehyde that allows immobilization of amino-containing molecules. The light dependence of the reactive functions allows the generation of photolithografically defined immobilization sites. Alternatively, introduction of photoaffinity moieties such as benzoyl benzoic acid ([480]; Figure 7.8iv), perfluorophenyl azides [25, 26, 553] or 4-[3-(trifluoromethyl)-3H -diaziridin-3-yl]benzoic acid (Figure 7.13; [91, 82, 252, 442]) generates highly reactive species on the surfaces, allowing addition to peptides or other molecules in close proximity upon irradiation with UV-light.
194 |
|
ULRICH REINEKE, JENS SCHNEIDER-MERGENER AND MIKE SCHUTKOWSKI |
||||||
|
|
O |
|
|
|
|
|
|
|
|
H |
|
|
O |
O |
|
|
|
|
N |
|
|
|
|
||
Fmoc |
|
OH |
|
H2N |
|
H2N |
|
|
|
|
+ |
+ |
|
||||
|
|
Ot Bu |
|
O |
OH |
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
i |
|
iii |
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
O |
|
|
O |
|
|
|
|
|
|
|
|
|
||
|
Fmoc N |
|
N |
O |
N |
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
Ot Bu |
|
H |
|
|
|
|
|
|
|
O |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ii |
iv |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
H |
|
|
|
HS |
|
|
O |
|
|
|
O |
|
H |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
N |
|
|
NH2 |
|
ligand |
|
O |
|
H2N |
|
|
vi |
spacer |
NH2 |
|||
O |
|
NH |
|
|
|
|
|
|
|
|
spacer |
|
|
|
|
|
|
|
|
ligand |
|
|
|
|
|
|
|
|
|
|
v |
|
|
|
|
|
|
|
|
|
H |
|
H |
O |
|
|
|
|
|
O |
N |
||
|
|
|
|
|
N |
N |
||
|
|
|
|
|
ligand |
3 |
O |
N |
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
O |
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
N |
N |
|
|
|
|
|
|
|
HN |
|
|
|
|
|
|
|
|
S |
H |
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
H |
|
O |
|
|
|
|
|
|
|
|
|
|
||
N |
O |
|
N |
|
NH2 |
|
|
|
ligand |
|
3 |
|
|
N |
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
O |
|
|
|
|
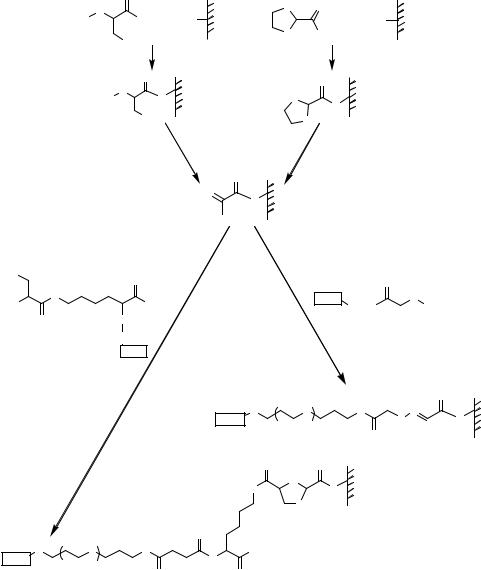
FIGURE 7.29. Preparation of aldehyde functionalized glass surfaces and chemoselective immobilization of peptides (ligands) containing either amino-oxyacetyl function or an N-terminal free cysteine [143]. i = aminopropyl silylated glass and Fmoc-Ser(OtBu)-OH, DIC, HOBt, dimethylformamide, 1.5 h; ii = 95% trifluoroacetic acid, 2 h, 20% piperidine in dimethylformamide followed by oxidation using 100 mM NaIO4 solution in PBS for 2 h, iii = protected glyoxylic acid, DIC, HOBt, dimethylformamide, 2 h, iv = 0.01 M HCl, room temperature, 2 h, v = chemoselective reaction with peptides containing an amino-oxyacetyl group, 250 mM NaOAc pH 5.2, 300 mM NaCl, vi = chemoselective reaction with ligands containing Lys(Cys)-amide at their carboxyl termini, 250 mM NaOAc pH 5.2, 300 mM NaCl.
PEPTIDE ARRAYS IN PROTEOMICS AND DRUG DISCOVERY |
|
|
|
195 |
||||||||||||||||||||
|
|
|
i |
|
|
|
|
ii |
|
|
|
O |
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
H2N |
|
|
|
O |
|
C |
|
N |
|
|
|
|
|
|
H2N |
C |
|
|||||||
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
N |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
H |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
||||||
|
|
|
|
|
|
|
|
ligand |
|
|
|
|
|
|
|
H |
||||||||
|
|
|
|
|
|
|
|
|
|
|
N |
|
N |
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
H |
|
H |
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
iii |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
ligand |
|
|
|
|
|
O |
|
N |
O |
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
C |
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
N |
|
|
|
|
N |
|
N |
|
|
|
N |
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
H |
|
|
|
|
H |
|
H |
|
|
|
H |
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H
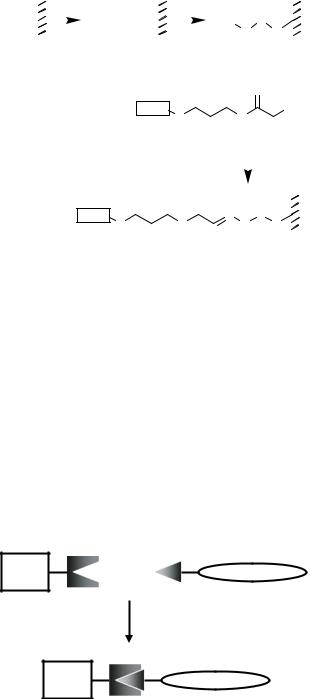
FIGURE 7.30. Preparation of semi-carbazide functionalized glass surfaces and chemoselective immobilization of glyoxylyl peptides [361]. i = aminopropyl silylated glass and triphosgen, DIEA, 1,2-dichloroethane; ii = Fmoc-hydrazide/dimethylformamide followed by treatment with piperidine/DBU in dimethylformamide; iii = chemoselective reaction with peptides containing an glyoxylyl group, 100 mM NaOAc pH 5.5.
7.2.3.3. Chemoselective Immobilization of Peptides Chemoselective immobilization reactions (Figure 7.31) are of particular interest in the preparation of peptide arrays because they allow control over both the orientation of the attached peptide and the density of the immobilized biomolecule. The ideal reaction is absolutely selective, avoiding any impairment caused by amino, carboxyl, mercapto, guanidino or hydroxyl functionalities from the peptides’ amino acid side chains. Additionally, the reaction has to be fast, minimizing the problems related to solvent evaporation during peptide microarray preparation. Recombinant tags represent a frequently used principle for chemoselective immobilization of proteins although they do not lead to the formation of a stable bond, for example via the oligohistidine tag or glutathione S-transferase conjugates. Alternatively, peptide immobilization using peptide tags (FLAG-tag, Myc-tag) or biotin moieties requires large and sensitive mediator proteins (antibodies, streptavidin).
support |
+ |
ligand |
|
|
chemoselective |
|
|
immobilization |
|
support |
ligand |
FIGURE 7.31. Principle of chemoselective immobilization.
196 |
ULRICH REINEKE, JENS SCHNEIDER-MERGENER AND MIKE SCHUTKOWSKI |
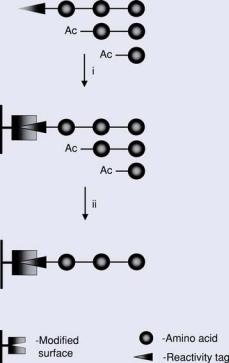
FIGURE 7.32. Reactivity purification during chemoselective immobilization. i = deposition of peptides mixtures and chemoselective immobilization: crude peptides normally contain truncated sequences resulting from incomplete coupling steps; introduced acetylations subsequent to every coupling reaction yield non-reactive truncated peptides together with the target peptide equipped with the reactivity tag; ii = washing steps subsequent to chemoselective immobilization yield purified covalently immobilized peptide.
One intrinsic advantage using chemoselective reactions is the formation of a covalent bond introduced as a result of reactivity purification (Figure 7.32). If the chemical moiety mediating the chemoselective reaction with the appropriately modified surface is attached to the N-terminus of the growing peptide, the peptide synthesis protocol can be modified to yield the target peptide equipped with the reactivity tag together with truncated, acetylated sequences resulting from incomplete coupling steps. Deposition of this mixture results in a covalent bond forming exclusively between the target peptide derivative and the surface. The chemically “inert” truncated sequences can be simply removed during subsequent washing steps. Thus, chemoselective reactions allow the generation of peptide arrays containing purified (free of truncated sequences) peptides.
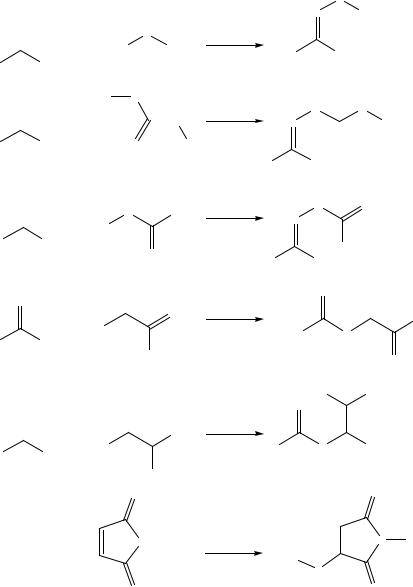
Classical chemoselective immobilization reactions (Figure 7.33) are reviewed by Lemieux and Bertozzi [307]. Chemoselective reactions used for the preparation of peptide arrays or which are suited for the oriented immobilization of peptides are presented in Table 7.3. Examples of maleimide-surface modifications of glass slides and titan [603] are given in Figure 7.1vi and Figure 7.2, respectively. Figure 7.3 illustrates the transformation
PEPTIDE ARRAYS IN PROTEOMICS AND DRUG DISCOVERY |
197 |
O
|
|
+ |
|
O |
A) |
|
H2N |
R2 |
|
|
|
RR1
|
O |
|
H2N |
NH |
|
|
|||
|
|
|
|
|
|
||||
B) |
|
|
|
|
+ |
|
|
|
NH |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
R |
|
|
|
|
R1 |
|
S |
|
R2 |
|
|
O |
|
H |
|
|
|
||
C) |
|
|
|
|
+ |
N |
|
|
R2 |
|
|
|
|
H2N |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
RR1
O
|
O |
D) |
O |
+ Br |
RS-
R1
|
|
O |
|
R2 |
||||||
|
|
|
|
|
|
|
||||
E) |
|
|
|
|
|
+ |
|
|
|
R3 |
|
|
|
|
|
|
|||||
|
R |
|
|
|
SR1 |
H2N |
||||
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
SH |
|
|
|
|
|
|
|
|
|
O |
||
G) |
R |
|
|
|
SH |
+ |
|
N |
|
R1 |
|
|
|
|
|
||||||
|
|
|
|
O |
||||||
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|||
O
N R2
RR1
H |
|
H |
||
N |
|
N |
||
N |
|
|
|
R2 |
|
|
|||
|
|
|
|
|
S
RR1
H
N O
N
R2
RR1
O
R1
RS
O
|
HS |
R3 |
|
O |
|
R |
N |
R2 |
|
H |
|
O
N R1
R
S
O
FIGURE 7.33. Chemical reactions useful for chemoselective immobilization of peptide derivatives.
of silicon wafers into mercapto-modified surfaces [308] allowing immobilization of maleimide-peptide conjugates. The formation of an oxime bond (Figure 7.29vi) using the chemoselective reaction between an amino-oxy-modified peptide derivative and an aldehyde surface has often been used in preparing peptide microarrays [143, 330, 395, 443, 484,

TABLE 7.3. Chemoselective reactions useful for the immobilization of peptides
educt |
educt (ligand) |
|
product |
|
|||||||
surface function |
functional group of ligand |
structure generated subsequent to |
|
||||||||
|
|
|
allowing chemoselective |
ligation or immobilization reaction |
|
||||||
|
|
|
reaction |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
reference |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
aldehyde/ketone |
amino-oxy-alkyl |
oxime |
[1, 143, 330, 395, 443, 484, 486, 503] |
||||||||
aldehyde/ketone |
ß-amino-thiol |
thiazolidine |
[143, 325] |
||||||||
ß-amino-thiol |
aldehyde |
thiazolidine |
[189] |
||||||||
hydrazide |
aldehyde |
hydrazone |
[167] |
||||||||
maleimide |
mercapto |
2-alkythio succinic imide |
[603] |
||||||||
α-halocarbonyl |
mercapto |
thioether |
[134] |
||||||||
mercapto |
maleimide |
2-alkythio succinic imide |
[67] |
||||||||
thioester |
ß-amino-thiol |
amide |
[99, 310, 311, 335, 538, 558]) |
||||||||
thiocarboxylate |
α-halocarbonyl |
thioester |
[496] |
||||||||
benzoquinone |
cyclopentadiene |
Diels-Alder-Adduct |
[607, Houseman, 2002] |
||||||||
Semicarbazide |
aldehyde |
semicarbazone |
[361, 425] |
||||||||
salicylhydroxamic acid (SHA) |
phenylboronic acid (PBA) |
PBA*SHA complex |
[530] |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
198
SCHUTKOWSKI MIKE AND MERGENER-SCHNEIDER JENS REINEKE, ULRICH
PEPTIDE ARRAYS IN PROTEOMICS AND DRUG DISCOVERY |
199 |
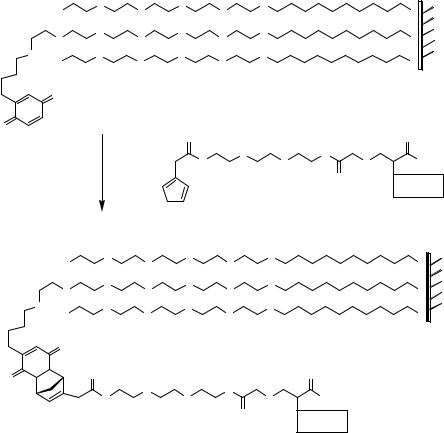
486, 503]. Alternatively, the reaction between aldehyde modified glass surfaces and peptides containing an N-terminal cysteine (Figure 7.29v) was also used successfully in peptide microarray preparation [143]. Melnyk and coworkers selected the reaction between semicarbazide glass slides and glyoxylyl peptide derivatives (Figure 7.30) to generate peptide microarrays [361]. It could be demonstrated that the native chemical ligation, introduced by Dawson et al. [99] is suited for effective attachment of peptides containing an N-terminal cysteine residue to thioester modified glass slides [310, 311, 558]. Finally, a special case of chemoselective immobilization of peptides is represented by the electrochemical copolymerization of pyrrole-modified peptides [200, 227, 328].
Recently, more sophisticated reactions have been introduced for oriented immobilization of peptide derivatives. A Diels-Alder reaction between benzoquinone groups on self-assembled monolayers and cyclopentadiene-peptide conjugates (Figure 7.34) led to
HO |
O |
O |
O |
|
S |
O |
O |
|
|
||
O |
O |
O |
O |
|
S |
O |
O |
|
|
||
O |
O |
O |
O |
|
S |
HO |
O |
O |
|
|
|
O |
|
|
|
|
|
O |
|
|
|
|
|
|
|
O |
|
H |
O |
|
|
O |
|
|
|
|
|
|
N |
|
|
|
|
N |
O |
S |
NH2 |
|
|
H |
|
O |
|
|
|
|
|
ligand |
|
|
|
|
|
|
|
O |
O |
O |
S |
|
HO |
O |
O |
|
|
O |
O |
O |
S |
|
O |
O |
O |
|
O |
O |
O |
O |
S |
|
HO |
O |
O |
|
|
O |
|
|
|
O |
O |
|
H |
O |
|
|
|
||
|
|
O |
|
|
|
|
N |
|
|
|
N |
O |
S |
NH2 |
|
H |
|
O |
|
|
|
|
ligand |
|
|
|
|
|
FIGURE 7.34. Chemoselective immobilization of cyclopentadiene-peptide conjugates to surface bound benzoquinone functions [218]. Diels-Alder reaction between self-assembled monolayers (prepared by reacting gold coated glass coverslips with a mixture of hydroquinone-oligo(ethylene glycol) and penta(ethylene glycol)-omega- mercaptoalkyl conjugates in methanol followed by oxidation to benzoquinones) and cyclopentadiene-peptide conjugates in aqueous solution yielding chemoselectively immobilized ligands (peptides).
200 |
ULRICH REINEKE, JENS SCHNEIDER-MERGENER AND MIKE SCHUTKOWSKI |
efficient covalent attachment of peptides to the surface at a density of 100 fmol/mm2 [218]. The formation of a 1:1 complex between surface-bound salicylhydroxamic acids and phenylboronic acid-conjugates was also used for chemoselective attachment of polypeptides to surfaces [530]. Moreover, selective cyclo-addition between biomolecules modified with an azido group and alkyne functions on the surface, resulting in the formation of a substituted 1H -[1,2,3]triazole [144], should be useful for peptide immobilization. A special case of immobilization is the selective interaction between surface-bound DNA molecules and peptide nucleic acid tags (PNA-tags). Schultz and coworkers used this interaction to assemble spatially addressed PNA-tagged peptidic protease inhibitors on an oligonucleotide microarray [596, 597].
7.2.4. Techniques for Array Production with Pre-Synthesized Peptides
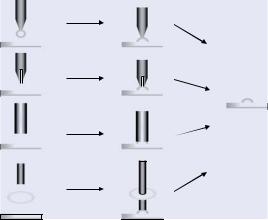
In general, the different technical solutions available for the production of peptide arrays starting from pre-synthesized peptides can be divided into two classes: contact and non-contact printing. During contact printing processes the sample is loaded onto the tip of a pin (Contact Tip Deposition Printing and Pin-and-Ring Printing), a pen of an atomic force microscope (dip-pen nanolithography (DPN), a small stamp (Micro Contact Printing, µCP), or into the needle of a microsyringe (Spotting) and subsequently deposited to the functionalized coherent surface by direct physical contact of the pin/pen/stamp/needle with the surface (Figures 7.35, 7.36). A special case of contact printing techniques is the so-called Micro-Wet-Printing (µWP) of the company Clondiag [137] useful for either immobilization of pre-synthesized peptides or the stepwise in situ synthesis of peptide arrays (Figure 7.36C).
In contrast, during non-contact printing procedures small droplets generated by piezoelectric or bubble-jet devices or by a microsyringe pump are sprayed with high speed onto a functionalized coherent surface without direct contact with the dispenser (Figure 7.37). This
A
B
C
D
FIGURE 7.35. Different principles of pin or capillary contact printing. A = solid pin printing; B = split pin printing, C = capillary printing; D = pin-and-ring printing.
PEPTIDE ARRAYS IN PROTEOMICS AND DRUG DISCOVERY |
201 |
CP
FN |
WP
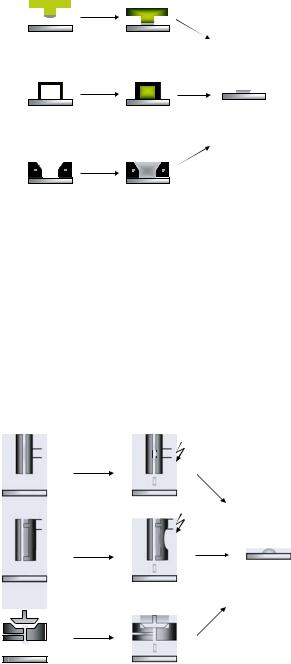
FIGURE 7.36. Different principles of contact printing. µCP = micro contact printing; µFN = micro fluidic networks; µWP = micro wet printing.
is a clear advantage because it avoids possible mechanical damage to either the dispenser or the sensitive surface.
7.2.4.1. Contact Printing The first step of Contact Tip Deposition Printing (Figure 7.35A) is dipping the tip of a solid pin into a sample solution. A defined amount of solution (depending on the material and on the shape and the dimensions of the pin) is adsorbed on the immersed tip. After moving to the appropriate printing position and establishing direct contact between the pin tip and the surface, a small spot of sample solution is generated on the surface (down to 50 µm spot size). If the same sample should be printed several times the pin has to be loaded again. However, so-called split pins were developed to circumvent
A
B
C
FIGURE 7.37. Different principles of non-contact printing. A = bubble jet: local heating generates a small bubble ejecting small droplets, B = piezoelectric device: compression of piezoelectric device generates a shock pulse in the fluidic chamber yielding a droplet, C = top-spot-printing: a microstamper ejects droplets simultaneously from array-like arranged micro-nozzles.
202 |
ULRICH REINEKE, JENS SCHNEIDER-MERGENER AND MIKE SCHUTKOWSKI |
this situation (Figure 7.35B). The small slot of the split pin represents a reservoir that is loaded with sample solution by capillary forces. Contact of the split pin with the surface deposits a defined part of the sample solution. After moving to another position of the surface an additional defined amount of sample solution can be deposited without reloading the pin.
Different devices for the production of arrays are on the market using up to 256 pins in parallel (Biorobotics multiple printhead) for simultaneous loading with sample solutions provided in 1536-well microtitre plates and sample deposition on a number of slides. Alternatively, capillaries either alone or connected to microsyringe pumps [508] can be used for the deposition of small sample droplets onto functionalized surfaces (Figure 7.35C). A unique technology is so-called Pin-and-Ring Printing (Figure 7.35D; [479]) making use of a small ring filled with spotting solution and a solid pin going through the sample solution, thereby transporting a fraction of the sample to the surface in a multiple mode.
Microscale placement of samples on (even curved) surfaces with submicron precision is possible using inked stamps of structured elastomers such as polyurethans, polyimides and poly(dimethylsiloxan) [36, 105, 229, 240, 280, 285, 314, 315, 319, 437, 600, 613]. This µCP technology (Figure 7.36A) has been reviewed extensively [600, 601]. The µCP technique has widespread application for printing of alkanethiols onto metal surfaces to form micro-patterned self-assembling monolayers. Positive µCP using pentaerythritol- tetrakis(3-mercaptopropionate) as an ink for wetting PDMS stamps was described recently [106]. Closely related to µCP is the recently introduced nanotransfer printing (nTP) technique, enabling the generation of complex patterns on functional materials with nanometer resolution in one step [333]. Alternatively, the µFN method is based on conformal contact between the functionalized surface and a micro-machined PDMS substrate (Figure 7.36B) forming a network of micro-channels, which can be filled with sample solution by capillary forces. The geometry of the µFN and the limited amount of sample molecules flowing inside the channels lead to a gradient of immobilized compounds.
Micro-structured masks are used for the synthesis of oligonucleotides by the µWP method (Figure 7.36C; www.clondiag.com). This mask is positioned with extremely high precision on the functionalized surface. The mask is connected to a channel system allowing either the deposition of pre-synthesized biomolecules or stepwise synthesis directly on the surfaces by application of appropriate activated building blocks. Squares of 1 µm size are theoretically possible with this technology, allowing the immobilization (or synthesis) of up to one million biomolecules per cm2.
A very special case of contact printing technologies is dip-pen nanolithography, making use of an inked atomic force microscope pen [6, 214, 302, 413]. Deposition of spots with diameters of around 50 nm onto gold or silicon oxide surfaces was demonstrated using this technology, resulting in a final density of more than 100,000 samples in an area of 100 µm by 100 µm [110].
7.2.4.2. Non-Contact Printing Several ink-jet modes of droplet generation have been used for the controlled delivery of small volumes of liquid to surfaces such as piezoelectric capillaries, piezoelectric cavities, nozzles with a thermal pressure transducer [14] and the nozzleless acoustic jet [541]. Of particular interest from the biochemical application standpoint is the acoustic jet method for droplet generation [132, 536] since the opportunity
