
Molecular and Cellular Signaling - Martin Beckerman
.pdf
40 2. The Control Layer
FIGURE 2.12. Ubiquitination and sumoylation: Conjugates are formed between lysine NH group and carbonyl groups at the C-terminal of ubiquitins and SUMO.
other proteins, and assists in localizing proteins to specific compartments within the cell.
2.16 Post-Translational Modifications to Histones
Post-translational modifications to histones are an important regulatory mechanism. Chromatin structure plays an important role in determining which genes are to be transcribed at any given moment in time. There are two forms of chromatin. Transcriptionally inactive chromatin, called heterochromatin, is tightly compacted. In heterochromatin, sites where transcription initiation and regulation take place are largely inaccessible to the proteins responsible for these actions. In contrast, transcriptionally active chromatin, or euchromatin, has a far more open shape. In euchromatin, sites where transcription factors and the transcription machinery bind are accessible to the responsible proteins.
As discussed earlier in this chapter, nucleosomes contain two each of several core histones. The amino terminals of these core histones extend out from the nucleosomes to form histone tails. These tails provide a means for other proteins to influence transcription. The histone tails provide sites for attachment of methyl groups, acetyl groups, and SUMO groups. The predominant targets of the post-translational modifications are the amino groups lying at the end of lysine side chains. The lysine residues, along with arginine residues, can be methylated, acetylated, sumoylated, or ubiquitinated. As shown in Figure 2.13, the tails of the histones are enriched in these residues, and thus supply multiple sites for attachment of these groups. Attachment of acetyl and other groups neutralizes the net positive charge on the tail regions, and this reduction weakens the attraction between the tails and the DNA. As a consequence the histone-DNA interactions are

2.16 Post-Translational Modifications to Histones |
41 |
FIGURE 2.13. Covalent modifications of histone tails: Shown are regulatory sites modified by acetylation (A), methylation (M), phosphorylation, or ubiquitination
(U). As is customary the sites are numbered in ascending order starting from the N-terminal. One-letter codes for the amino acids are arginine (R), lysine (K), and serine (S). (Threeand one-letter abbreviations for the amino acids are listed in Table 4.1.)
lessened allowing for a greater access of the DNA to transcription regulators. Similarly, addition of net negative charge through phosphorylation on serine residues also serves to decondense chromatin.
Many of the proteins that act in a supporting role to help activate or suppress transcription interact directly with the histones rather than with the DNA. Some of these enzymes function as histone acetyltransferases (HATs) or alternatively as histone deacetylases (HDACs), adding or removing acetyl groups from histone tails, using acetyl coenzyme A (acetyl-CoA) as the donor. Other coregulatory enzymes operate in a similar manner, adding or removing methyl groups or phosphoryl groups or SUMO groups or ubiquitin groups.

42 2. The Control Layer
General References
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, and Walker P [2002]. Molecular Biology of the Cell, 4th edition. New York: Garland Publishers.
Matthews CK, van Holde KE, and Ahem KG [2000]. Biochemistry, 3rd edition. San Francisco: Pearson Benjamin Cummings.
Stryer L [1995]. Biochemistry, 4th edition. New York: W.H. Freeman and Company.
References and Further Reading
Chromatin and the Nucleosome
Harp JM, et al. [2000]. Asymmetries in the nucleosome core particle at 2.5 Å resolution. Acta Cryst., D56: 1513–1534.
Kornberg RD, and Lorch YL [1999]. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell, 98: 285–294.
Nuclear Organization
Lamond AI, and Earnshaw WC [1998]. Structure and function in the nucleus. Science, 280: 547–553.
Lewis JD, and Tollervey D [2000]. Like attracts like: Getting RNA processing together in the nucleus. Science, 288: 1385–1389.
Chromosome Organization
Cremer T, and Cremer C [2001]. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat. Rev. Genet., 2: 292–301.
Manuelidis L [1990]. A view of interphase chromosomes. Science, 250: 1533–1540.
Protein Organization
Hovmöller S, Zhou T, and Ohlson T [2002]. Conformations of amino acids in proteins. Acta Cryst., D58: 768–776.
Kleywegt GJ, and Jones TA [1996]. Phi/Psi-chology: Ramachandran revisited. Structure, 4: 1395–1400.
Post-Translational Modifications
Fortini ME [2000]. Fringe benefits to carbohydrates. Nature, 406: 357–358, and references cited therein.
McLaughlin S, and Aderem A [1995]. The myristoyl-electrostatic switch: A modulator of reversible protein-membrane interactions. Trends Biochem. Sci., 20: 272–276.
Milligan G, Parenti M, and Magee AI [1995]. The dynamical role of palmitoylation in signal transduction. Trends Biochem. Sci., 20: 181–186.
Peschon JJ, et al. [1998]. An essential role for ectodomain shedding in mammalian development. Science, 282: 1281–1284.
Resh MD [1999]. Fatty acylation of proteins: New insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta, 1451: 1–16.

Problems 43
Regulated Proteolysis
Brown MS, et al. [2000]. Regulated intramembrane proteolysis: A control mechanism conserved from bacteria to humans. Cell, 100: 391–398.
Maniatis T [1999]. A ubiquitin ligase complex essential for the NF-B, Wnt/Wingless, and Hedgehog signaling pathways. Genes Dev., 13: 505–510.
Townsley FM, and Ruderman JV [1998]. Proteolytic ratchets that control progression through mitosis. Trends Cell Biol., 8: 238–244.
Sumoylation
Müller S, et al. [2001]. SUMO, ubiquitin’s mysterious cousin. Nature Rev. Mol. Cell Biol., 2: 202–210.
Histone Modifications
Grunstein M [1997]. Histone acetylation in chromatin structure and transcription. Nature, 389: 349–352.
Jenuwein T, and Allis CD [2001]. Translating the histone code. Science, 293: 1074– 1080.
Problems
These problems require use of a PC to run the Protein Explorer, the free software used to model the Src protein as shown in Figure 2.7. The first step is to install “Chime” to enable your browser to talk to the Protein Explorer. Then bring up the Protein Explorer. (You may want to save the URL under your “Favorites” pull-down.)
2.1Bring up the atomic coordinates for the Src protein displayed in Figure 2.7. Its Protein Data Bank (PDB) accession number is 2src. Next remove the water molecules and ligands leaving just Src. Then display the image in the form shown in Figure 2.7. Hint: Go to “Quick Views” and use the “Display” feature to bring up the “cartoon” depiction of the secondary structure. Then drag on the image to rotate into the orientation presented in Figure 2.7.
2.2Click on “Mol Info” and then the “Header” button to bring up additional information about the protein Src. Note the list of amino acids comprising the primary structure.
2.3Returning to the image of the Src protein. Bring up a “backbone” display of the protein. What does this image show?
2.4Now, bring up a “ball-and-stick” model of Src. What do the balls represent and what do the sticks denote? Click on some of the balls shown in the image. What happens when this is done?
2.5Change the display to a “space-fill” model. What do the spheres now mean; that is, what do their radii represent? How is hydrogen treated?

3
Exploring Protein Structure and Function
A variety of methods have been developed that enable researchers to study the structure and function of proteins at the atomic, molecular, intermolecular and cellular levels. Some methods exploit differences in charge and mass of the proteins in order to distinguish one kind of protein from another. Other methods exploit the many kinds of interactions occurring between electromagnetic radiation and biomolecules. Depending on the wavelength and the properties of the target material, electromagnetic radiation will be scattered and diffracted, absorbed and emitted. In all, an ensemble of methods is used in the laboratory not only to explore protein structure, but also to investigate posttranslational modifications, intermolecular interactions, cellular localization, and pleiotropy.
The preeminent methods for exploring the shape and internal structure of proteins at atomic level detail are X-ray crystallography and nuclear magnetic resonance (NMR). These techniques provide detailed threedimensional information on how the proteins are organized into their functionally distinct domains and motifs, and what happens when one protein binds to another. They identify which amino acid residues are critical for protein-protein and protein-DNA binding, and they reveal the functional consequences of mutations of specific amino acid residues. As will be seen in later chapters, proteins involved in intracellular signaling often possess catalytic domains that stimulate the transfer of phosphoryl groups from one protein to another. X-ray crystallography and NMR show how these catalysts work, and how their activities are regulated.
The goal of techniques such as gel electrophoresis and DNA microarrays is to examine which proteins are expressed at higher levels and which ones at lower levels in response to specific kinds of signaling events and conditions. That is, they provide intermolecular and cellular level details. The mass spectrograph, often used in conjunction with these methods, permits the researcher to determine with high resolution the masses of proteins that have been isolated by gel electrophoresis and the microarrays. Another method, the yeast two-hybrid method, has become the leading method for determining which sets of proteins interact with one another to form the
45

46 3. Exploring Protein Structure and Function
TABLE 3.1. Methods using electromagnetic interactions to explore protein structure and interactions.
Experimental method |
Level of detail |
Process |
X-ray crystallography |
Atomic |
X-ray diffraction |
Circular dichroism |
Molecular |
Absorption of polarized |
|
|
UV light |
Fluorescence resonance energy transfer |
Intermolecular |
Visible light absorption |
|
|
and emission |
IR and Raman spectroscopy |
Molecular |
Absorption (IR) and |
|
|
scattering (Raman) of IR |
|
|
light |
NMR spectroscopy |
Atomic |
Nuclear spin flips |
|
|
|
TABLE 3.2. Physical methods used to explore protein structure and interactions.
Experimental method |
Level of detail |
Process |
Yeast two-hybrid |
Intermolecular |
Protein-protein interactions |
Gel electrophoresis |
Molecular, Intermolecular |
Mass/charge separation |
Mass spectrograph |
Molecular |
Mass/charge separation |
DNA microarrays |
Cellular |
Complementary base-pairing |
|
|
|
signaling pathways in the cell. And another method, fluorescence resonance energy transfer, is used to explore protein interactions and view the movements of the signaling proteins in the cell. All of the methods listed in Tables 3.1 and 3.2 will be discussed in this chapter, some to a greater extent than others. The discussion will start with a review of the electromagnetic spectrum and how electromagnetic waves interact with matter. X-ray crystallography, NMR, and FRET will be explored next, followed by discussions of the physical methods.
3.1Interaction of Electromagnetic Radiation with Matter
Electromagnetic radiation interacts with matter in a variety of ways. Recall that the wavelength and frequency of an electromagnetic wave are inversely proportional to one another, and the speed of light is the constant of proportionality. That is,
c |
|
|
n = l |
, |
(3.1) |
where l (cm) is the wavelength, n (cycles/s) denotes the frequency, and c represents the speed of light, equal to 2.99 ¥ 1010 cm/s. Electromagnetic radi-

3.1 Interaction of Electromagnetic Radiation with Matter |
47 |
ation is quantized into discrete packets called photons. The energy E of each packet is given by Planck’s formula:
E = hn, |
(3.2) |
where h = 6.626 ¥ 10-27 erg-sec is Planck’s constant. According to Eqs. (3.1) and (3.2), as the frequency increases, or equivalently the wavelength decreases, the photon energy goes up.
Electromagnetic radiation is not limited to a narrow range of wavelengths, but rather spans a broad range of wavelengths from less than a nanometer to more than a meter. The shortest waves are the gamma rays (g-rays) emitted by atomic nuclei, and the longest waves are radio waves emitted by charged particles as they move back and forth in, for example, interstellar gases. The continuum of different kinds of radiation, each characterized by a unique wavelength, is known as the electromagnetic spectrum. The middle portion of the electromagnetic spectrum contains the infrared and ultraviolet regions with the visible range sandwiched in between. These three regimes are the most important portion of the spectrum with respect to biological systems.
According to the formulas just presented, as wavelengths decrease, photon energies increase. The energies corresponding to the different wavelength regimes are presented in Figure 3.1 in units of kcal/mol to facilitate comparison to familiar bonding energies, which are usually given in these units—the energies of covalent bonding are on the order of 100 kcal/mol. Noncovalent interactions such as hydrogen bonds have energies in the range 1 to 10 kcal/mol, and thermal energies are roughly 0.6 kcal/mol.
FIGURE 3.1. Electromagnetic spectrum: Shown in the upper portion of the figure are the types of radiation emitted. The kinds of transitions, or motions, that absorb and emit the radiation are shown in the lower part of the figure. Vertical dashed lines delineate the boundaries between the different regimes. The actual boundaries between radio waves and microwaves, and between ultraviolet and X-rays, are not sharp but instead the regimes merge into one another.
48 3. Exploring Protein Structure and Function
Molecules are not static entities but instead are in constant thermal motion, gaining and losing energy through random collisions with other molecules. The kinetic energy absorbed in the collisions is converted into vibrations and rotations about bond angles. These so-called collective modes can also be excited when the atoms and molecules absorb radiation in the appropriate wavelength range. The correspondence between wavelength regimes of electromagnetic radiation and protein motions (transitions) that produce the radiation is presented in the lower portion of Figure 3.1.
Recall that electrons move in specific atomic and molecular orbits, each with a well-defined energy. The lowest energy state of the electrons in their various orbits around a nucleus is called the ground state, and all others are referred to as excited states. Besides inducing vibrational and rotational activity, electrons can transition into excited states when radiation of the correct wavelength is absorbed. Electromagnetic radiation of well-defined energies is involved whenever electrons, atoms, and molecules undergo transitions from one state to another, either higher or lower. The energy of the absorbed and emitted radiation is equal to the difference in energies between the two energy levels (states), and its frequency (wavelength) is given by the following form of Planck’s formula:
E2 - E1 = DE = hn. |
(3.3) |
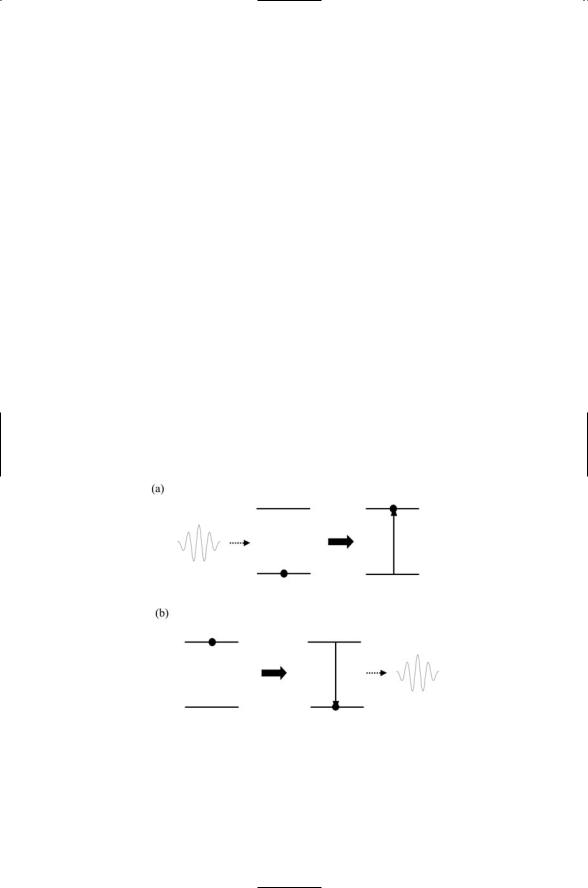
The absorption and emission of photons is depicted in Figure 3.2.
FIGURE 3.2. Absorption and emission of electromagnetic radiation: Horizontal lines represent energy levels of a simplified molecule possessing just a single ground state and a single excited state. From left to right: (a) Absorption—The molecule, initially in its ground state, absorbs a photon and undergoes a transition to an excited state.
(b) Emission—The molecule, initially in its excited state, undergoes a transition to the ground state and emits a photon in the process.

3.3 Protein Structure via X-Ray Crystallography |
49 |
3.2 Biomolecule Absorption and Emission Spectra
Biomolecules such as amino acids, peptides, and proteins absorb and scatter light in the ultraviolet and visible portions of the electromagnetic spectrum. Many of these biomoleucles selectively absorb light of particular wavelengths while scattering light at other wavelengths. These molecules act as pigments, imparting color to the materials in which they reside. The specific groups of atoms responsible for these absorption properties are known as chromophores, or “color bringers.” The chlorophylls are prominent examples of chromophore-bearing molecules. They selectively absorb light in the blue and red portions of the spectrum, and scatter light in the green range. The result is the pronounced green color of plants.
Another common example of a biomolecule with striking chromatic properties is hemoglobin. Its absorption/scattering properties impart a red color to fully oxygenated blood while conveying a bluish tint to deoxygenated blood.Those electrons in a chromophore responsible for the light absorption are sensitive to their local environments, especially the presence of nearby charged groups. In the case of hemoglobin, the heme group forming the chromophore is sensitive to the presence or absence of bound oxygen atoms.
Photoreceptors form another class of chromophore-bearing proteins. The class includes ospins found in the mammalian retina along with phytochromes and cryptochromes, which enable bacteria, plants, and animals to adapt their cellular responses to local lighting conditions and undergo circadian rhythms. Opsins will be discussed in Chapter 12. Phytochromes and cryptochromes will be explored in Chapter 7. Protein chromophores can be built about the peptide bond, or about side chains, or upon prosthetic groups covalently attached chains that are nominally not part of the protein. Examples of prosthetic groups operating as chromophores are the heme groups and opsins.
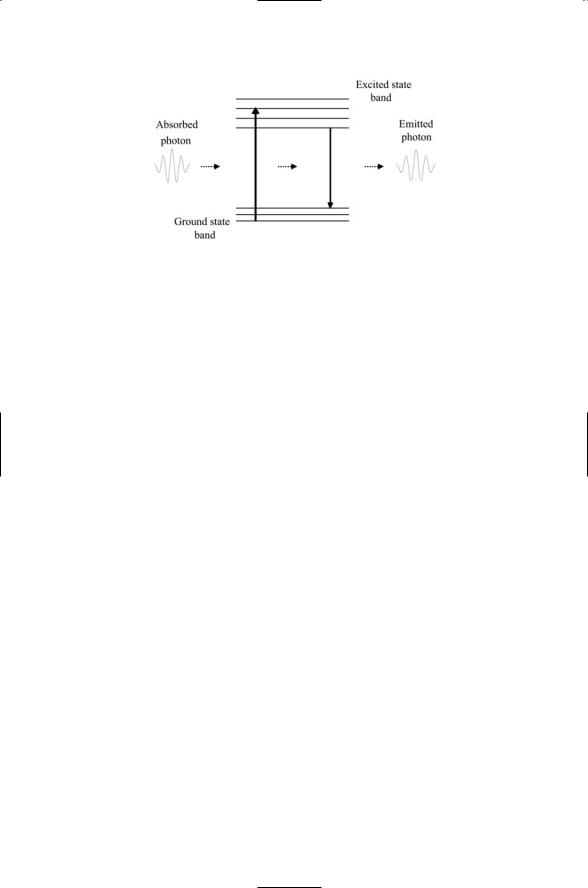
When atomic groups absorb light, electrons are promoted into excited states (orbits). One of three things can then happen. The electrons may deexcite to the ground state without emitting radiation in a process called internal conversion. Alternatively, the excess energy may be lost through emission of photons having energies less than those of the absorbed light,with the remaining energy lost in a radiationless manner. If this happens rapidly, in a time scale of nanoseconds, the process is referred to as fluorescence (Figure 3.3), and the chromophores are called fluorophores. If, on the other hand, the lifetime of the excited state is long-lived, in the millisecond to second range, the process of absorbing and then emitting light is called phosphorescence.
3.3 Protein Structure via X-Ray Crystallography
Because of their short wavelength, X-rays can be used to explore the arrangements of individual atoms in proteins and other biomolecules. X- rays are produced whenever swiftly moving electrons strike a solid target.
50 3. Exploring Protein Structure and Function
FIGURE 3.3. Schematic depiction of fluorescence: Shown are sets of related states, or bands, along with vertical arrows denoting transitions between states. From left to right, a packet of electromagnetic radiation is absorbed by a fluorophore, producing a transition from the ground state to an excited state. Shortly thereafter, the fluorophore deexcites back to the ground state, emitting a photon with a slightly lower energy and longer wavelength. Energy not carried off by the emitted photon is lost through radiationless transitions between vibrational states within the excited state band and within the ground state band.
In an X-ray tube, a beam of electrons is generated that strikes a metallic anode (typically copper) to produce an X-ray beam. Two types of X-rays are produced—characteristic X-rays that generate a line spectrum, containing a set of narrow strong peaks, and a smooth spectrum of continuum X-rays produced by coulombic interactions between the electron beam and the positive-charged nuclei (called bremsstrahlung, or braking radiation). In X-ray studies, characteristic Ka X-rays produced by transitions among inner shell electrons are typically used. These have a well-defined wavelength of about 1.5 Å, comparable to atom-atom bond lengths.
X-rays are scattered by the electron clouds of atoms, particularly by tightly bound electrons near the center of atoms, with no change in wavelength and no change in phase. Light scattered from single atoms is too weak to observe, but the amount of light can be amplified using purified crystals. In a crystal, large numbers of identical molecules are arranged in a regular lattice. Light passing through a crystal will be scattered in a variety of directions. Spherical wavelets scattered by different atoms will interfere, some constructively and some destructively. For certain wavelengths and scattering directions, the wavelets will be in phase to produce strong constructive interference. Constructive interference taking place between light waves as they are scattered off of the atoms serves to amplify the light, producing a characteristic pattern of light spots and dark areas, a diffraction pattern, that can be seen and analyzed to yield information on how the atoms are arranged.
