
Covalent analogues of DNA base-pairs and triplets . Part 2[c] Synthesis and cytostatic activity of bis(purin-6-yl)acetylenes, -diacetylenes and related compounds
.pdf
Bioorganic & Medicinal Chemistry Letters 12 (2002) 1055–1058
Covalent Analogues of DNA Base-Pairs and Triplets. Part 2:y Synthesis and Cytostatic Activity of Bis(purin-6-yl)acetylenes, -diacetylenes and Related Compounds
Michal Hocek* and Ivan Votruba
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo na´m. 2, CZ-16610 Prague 6, Czech Republic
Received 10 December 2001; revised 24 January 2002; accepted 28 January 2002
Abstract—The title bis(purin-6-yl)acetylenes, -diacetylenes, -ethylenes and -ethanes were prepared as covalent base-pair analogues starting from 6-ethynylpurines and 6-iodopurines by cross-coupling and homo-coupling reactions and hydrogenations. The bis(purin-6-yl)acetylenes and -diacetylenes exhibited significant cytostatic activity in vitro (IC50=0.4–1.0 mmol/l). # 2002 Elsevier Science Ltd. All rights reserved.
The e ect of many clinically used antitumor agents is based on DNA cross-linking2 or on intercalation3 to DNA. Numerous models and analogues of Watson– Crick base pairs consisting of annelated4 or crosslinked5 purine and pyrimidine heterocycles or even more simple aromatic rings6,7 have been prepared. Recently, also the first covalently linked analogues of Hoogsteen triplets were prepared1 in our laboratory. Such base-pairs/triplets analogues may interact with DNA (e.g., by intercalation); if incorporated into single stranded DNA, they are complementary to abasic site of a damaged DNA strand; or alternatively, if incorporated to duplex, they form permanent cross-links.
A number of diverse purine–purine conjugates containing linkage (9–9, 8–8, 9–8, 9–7, 9–6 and 6–6) of various lengths, including doubleand triple-linked purinophanes, have been prepared8 in order to study the p–p stacking of purine bases. The purine–purine dimers with a 6,60-pyridine-2,6-bis(carboxamido) linker have been recently prepared9 to study its H-bonding properties as potential artificial receptors. Methylene N6,N60- linked-adenine-adenine dimers are formed by the reaction of carcinogenic formaldehyde with DNA, which was proved by independent synthesis10 of the products.
Avariety of other N6,N60-linked-adenine-adenine
*Corresponding author. Tel.: +420-2-2018-324; fax: +420-2-33331- 271; e-mail: hocek@uochb.cas.cz
yFor Part I, See ref 1.
dimers, trimers and tetramers with the linkers of various lengths were prepared11 and exhibited diverse types of biological activity (inhibition of adenosine kinase, ribosomal peptidyltransferase, etc.).
Purines bearing carbon substituents in positions 2 or 6 possess a broad spectrum of biological activity. Thus, 6-methylpurine is highly cytotoxic,12 while 2-alkynyl- adenosines are an important class of adenosine receptors agonists.13 Recently, a cytokinin activity of 6-(arylalky- nyl)-, 6-(arylalkenyl)- and 6-(arylalkyl)purines,14 a cytostatic activity of 6-(trifluoromethyl)purine riboside15 and of 6-arylpurine ribonucleosides,16 a corticotropin-releas- ing hormone antagonist activity of some 2,8,9-trisub- stituted-6-arylpurines17 and an antimycobacterial activity of 9-benzyl-6-arylpurines18 were also reported.
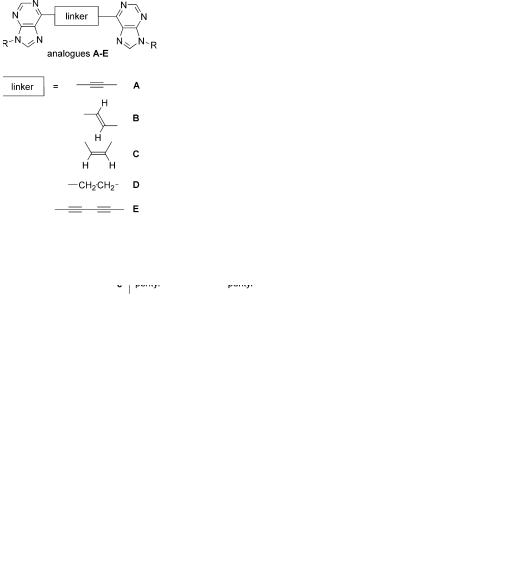
A combination of the unique structural features of the above mentioned classes of compounds led us to the design of a new group of base-pair analogues A–E (Chart 1) based on covalent purine–purine conjugates linked through positions 6 and 60 by carbon linkages. The linkers di er from rigid linear acetylene-linkage, bend E- or Z-vinylene-linkers to conformationally flexible saturated ethylene-spacer. Such carbon linkers connected to carbon atoms of the heterocycles are expected to be stable towards enzymatic degradation. Apparently, such ‘extended’ analogues are larger than the parent Watson–Crick base-pairs but on the other hand they could be capable of intercalation within the DNA
0960-894X/02/$ - see front matter # 2002 Elsevier Science Ltd. All rights reserved. PII: S0960-894X(02)00077- X
1056 |
M. Hocek, I. Votruba / Bioorg. Med. Chem. Lett. 12 (2002) 1055–1058 |
replication fork and thus inhibit its synthesis ‘de novo’ and cell division. This is a preliminary communication reporting their synthesis and cytostatic activity.
Chemistry
The synthesis of the target compounds was based on standard acetylene chemistry (Scheme 1) using the 9-benzyl-6-ethynylpurine19 (1a) as a key starting compound. Attempted Sonogashira reaction of this compound with 9-benzyl-6-iodopurine (2a) in presence of CuI, Pd(PPh3)4 and Et3N in DMF did not give the expected bis(purin-6-yl)acetylene 5a but its partly reduced E-ethylene derivative 3a in 27% yield.20 The formation of this product could be explained by a reductive addition21 of the iodopurine 2a on the acetylene 1a. Catalytic hydrogenation of the ethylene derivative 3a on Pd/C gave the fully saturated ethane derivative 4a in good yield of 80%.
Chart 1.
For the synthesis of the acetylene derivative 5a, an alternative method based on recently published procedure22 has been used. The reaction of the 6-iodopurine 2a with the terminal acetylene 1a was made in presence of tetrabutylammonium fluoride (TBAF) as base, catalytic amount of CuI and PdCl2(PPh3)2 in THF at room temperature to give23 the desired acetylene 5a in good yield of 57%. This approach has also been used for the synthesis of other related symmetrically and asymmetrically disubstituted acetylenes 5b and 5c di ering by the substituent in the positions 9 and 90 starting from the appropriate iodopurines 2a and 2c and ethynylpurines 1b and 1c. Catalytic hydrogenation of the acetylene 5a on Lindlar catalyst a orded the complementary Z- ethylene derivative 6a in a low yield of 11% accompanied by the fully saturated compound 4a (19%). Oxidative homo-coupling24 of the terminal acetylenes 1a–1c in presence of CuCl and TMEDA25 a orded26 the 1,4- bis(purin-6-yl)diacetylenes 7a–7c in good yields of 50–60%.
Cytostatic activity evaluation
The target base-pair analogues 3–7 were tested27 on their in vitro inhibition of the cell growth in the following cell cultures: mouse leukemia L1210 cells (ATCC CCL 219); murine L929 cells (ATCC CCL 1); human cervix carcinoma HeLa S3 cells (ATCC CCL 2.2); and human T lymphoblastoid CCRF-CEM cell line (ATCC CCL 119). The results (Table 1) show that while the bis(purinyl)acetylenes 5a–5c and -diacetylenes 7a–7c exhibited a significant cytostatic e ect in these assays (IC50=0.3–15 mM), the partly and fully saturated derivatives bearing vinylene and ethylene linkers, compounds 3a, 4a and 6a, were entirely inactive. The nature of substituents in the positions 9 of the purine rings has only little e ect on the activity (derivatives a–c in each series of active compounds). Among the assays tested, the T- lymphoblastoid CCRF-CEM and leukemia L1210 cells were the most sensitive to the action of these compounds.
Scheme 1.

M. Hocek, I. Votruba / Bioorg. Med. Chem. Lett. 12 (2002) 1055–1058 |
1057 |
Table 1. Cytostatic activity data for compounds 3–7
Compd |
|
|
|
IC50 (mM)a |
|
|
|
|
|
|
|
|
|
|
|||
|
L1210 |
|
L929 |
HeLa S3 |
CCRF-CEM |
|||
|
|
|
|
|
|
|
|
|
FUDRb |
<0.02 |
( c0.002) |
|
>25 |
>25 |
0.5 |
( 0.04) |
|
3a |
|
na |
|
na |
|
na |
|
na |
4a |
|
na |
|
na |
|
na |
|
na |
5a |
1.8 |
( 0.17) |
5.3 |
( 0.6) |
|
na |
0.9 |
( 0.08) |
5b |
0.9 |
( 0.08) |
1.9 |
( 0.2) |
6.0 |
( 0.6) |
0.32 |
( 0.07) |
5c |
1.5 |
( 0.12) |
3.0 |
( 0.3) 15.0 ( 1.8) |
0.36 |
( 0.03) |
||
6a |
|
na |
|
na |
|
na |
|
na |
7a |
0.37 |
( 0.03) |
6.3 |
( 0.5) |
|
na |
0.43 |
( 0.03) |
7b |
0.7 |
( 0.05) |
1.0 |
( 0.12) 13.3 |
( 1.3) 0.58 ( 0.04) |
|||
7c |
0.5 |
( 0.07) |
1.3 |
( 0.11) |
15.0 |
( 1.1) |
0.37 |
( 0.03) |
aValues are means of four experiments, standard deviation is given in parentheses.
b1-(b-d-2-deoxy-erythro-pentofuranosyl)-5-fluorouracil.
cna, not active (inhibition of cell growth at 10 mM was lower than 20%).
In conclusion, the substituted bis(purin-6-yl)acetylenes and -diacetylenes represent a novel class of antineoplastic compounds. They are characterized by a rigid linear (acetylene or diacetylene) linker between the two purine rings. It is not yet clear whether the role of this spacer is just in being a linear linker of certain steric parameters or whether the alkyne forms covalent or non-covalent adducts with the target cell system (DNA or enzyme). Though we suspect that the intercalation or other interaction with DNA might be the mode of action of this class of compounds, these problems remain the subjects for further investigation.
Acknowledgements
This work is a part of a research project Z4055905. It was supported by the Grant Agency of the Czech Republic (grant No. 203/00/0036). NMR spectra were measured and interpreted by Dr. Hana Dvorˇa´kova´ (Prague Institute of Chemical Technology).
References and Notes
1.Hocek, M.; Stara´, I. G.; Stary´, I.; Dvorˇa´kova´, H. Tetrahedron Lett. 2001, 42, 519.
2.Review: Rajski, S. R.; Williams, R. M. Chem. Rev. 1998, 98, 2723.
3.Review: Baguley, B. C. Anti-Cancer Drug. Des. 1991, 3, 1.
4.Bhat, B.; Leonard, N. J.; Robinson, H.; Wang, A. H. J. J. Am. Chem. Soc. 1996, 118, 10744 and references cited therein.
5.(a) Nagatsugi, F.; Uemura, K.; Nakashima, S.; Maeda, M.; Sasaki, S. Tetrahedron Lett. 1995, 36, 421. (b) Nagatsugi, F.; Uemura, K.; Nakashima, S.; Maeda, M.; Sasaki, S. Tetrahedron 1997, 53, 3035. (c) Nagatsugi, F.; Kawasaki, T.; Usui, D.; Maeda, M.; Sasaki, S. J. Am. Chem. Soc. 1999, 121, 6753.
(d) Nagatsugi, F.; Usui, D.; Kawasaki, T.; Maeda, M.; Sasaki, S. Bioorg. Med. Chem. Lett. 2001, 11, 343.
6.(a) Quiao, X.; Kishi, Y. Angew. Chem., Int. Ed. Engl. 1999,
38, 928. (b) Qiu, Y. L.; Li, H. Y.; Topalov, G.; Kishi, Y. Tetrahedron Lett. 2000, 41, 9425. (c) Li, H. Y.; Qiu, Y. L.; Moyroud, E.; Kishi, Y. Angew. Chem., Int. Ed. Engl. 2001, 40, 1471. (d) Li, H. Y.; Qiu, Y. L.; Kishi, Y. Chem. Bio. Chem. 2001, 2, 371.
7.(a) Ogawa, A. K.; Abou-Zied, O. K.; Tsui, V.; Jimenez, R.; Case, D. A.; Romesberg, F. E. J. Am. Chem. Soc. 2000, 122, 9917. (b) Abou-Zied, O. K.; Jimenez, R.; Romesberg, F. E.
J. Am. Chem. Soc. 2001, 123, 4613.
8.(a) Leonard, N. J.; Ito, K. J. Am. Chem. Soc. 1973, 95, 4010. (b) Akahori, K.; Hama, F.; Sakata, Y.; Misumi, S. Tetrahedron Lett. 1984, 25, 2379. (c) Seyama, F.; Akahori, K.; Sakata, Y.; Misumi, S.; Aida, M.; Nagata, C. J. Am. Chem. Soc. 1988, 110, 2192.
9.Crisp, G. T.; Jiang, Y. L. Tetrahedron 1999, 55, 549.
10.Chaw, Y. F. M.; Crane, L. E.; Lange, P.; Shapiro, R.
Biochemistry 1980, 19, 5525.
ˇ
11. (a) Zemlicˇka, J.; Owens, J. J. Org. Chem. 1977, 42, 517. (b)
ˇ ˇ
Bhuta, P.; Li, C.; Zemlicka, J. Biochem. Biophys. Res. Com-
ˇ ˇ
mun. 1977, 77, 1237. (c) Zemlicka, J. Biochemistry 1980, 19,
ˇ
163. (d) Murata, M.; Bhuta, P.; Owens, J.; Zemlicˇka, J. J.
ˇ
Med. Chem. 1980, 23, 781. (e) Zemlicˇka, J. Tetrahedron Lett.
1984, 25, 5619. (f) Agathocleous, D. C.; Page, P. C. B.; Cosstick, R.; Galpin, I. J.; McLennan, A. G.; Prescott, M. Tetrahedron 1990, 46, 2047.
12.Montgomery, J. A.; Hewson, K. J. Med. Chem. 1968, 11,
13.Review: Mu¨ller, C. E. Curr. Med. Chem. 2000, 7, 1269.
14.Brathe, A.; Gundersen, L. L.; Rise, F.; Eriksen, A. B.;
Vollsnes, A. V.; Wang, L. N. Tetrahedron 1999, 55, 211.
15.Hockova´, D.; Hocek, M.; Dvorˇa´kova´, H.; Votruba, I.
Tetrahedron 1999, 55, 11109.
16.(a) Hocek, M.; Holy´, A.; Votruba, I.; Dvorˇa´kova´, H. J. Med. Chem. 2000, 43, 1817. (b) Hocek, M.; Holy´, A.; Votruba, I.; Dvorˇa´kova´, H. Collect. Czech. Chem. Commun. 2000, 65, 1683. (c) Hocek, M.; Holy´, A.; Votruba, I.; Dvorˇa´kova´, H.
Collect. Czech. Chem. Commun. 2001, 66, 483.
17.Cocuzza, A. J.; Chidester, D. R.; Culp, S.; Fitzgerald, L.; Gilligan, P. Bioorg. Med. Chem. Lett. 1999, 9, 1063.
18.Bakkestuen, A. K.; Gundersen, L. L.; Langli, G.; Liu, F.; Nolsøe, J. M. J. Bioorg. Med. Chem. Lett. 2000, 10, 1207.
19.Tanji, K.; Higashino, T. Chem. Pharm. Bull. 1988, 36, 1935.
20.Its E-configuration has been assigned based on NOE of its cycloadduct with cyclopentadiene. The details are beyond the scope of this communication and will appear in a forthcoming full-paper.
21.An analogous reductive addition has been recently observed for the reaction of aryl halides with substituted acetylenes in presence of NaOMe: Wu, M.-J.; Wei, L.-M.; Lin, F.-C.; Leou, S.-P.; Wei, L.-L. Tetrahedron 2001, 57, 7839. In our case, apparently Et3N was the reducing agent.
22.Mori, A.; Shimada, T.; Kondo, T.; Sekiguchi, A. Synlett 2001, 649.
23.Typical procedure: A degassed 0.165 M solution of TBAF trihydrate in THF (24 mL, 4 mmol) was added dropwise to an argon purged flask containing 1a (407 mg, 1.67 mmol), 2a (560
mg, 1.67 mmol), CuI (60 mg, 0.3 mmol), PdCl2(PPh3)2 (100 mg, 0.14 mmol) at ambient temperature and the mixture was stirred for 4 h. The solvent was evaporated and the residue was chromatographed on silica gel (200 g, ethyl acetate–light
petroleum 1:1) to give the 1,2-bis(9-benzylpurin-6-yl)acetylene (5a) (420 mg, 57%), mp 254–257 C (CH2Cl2/heptane), FAB
MS, m/z (rel.%): 443 (5) [M+H], 91 (100). IR (CHCl3): n=1583, 1448, 1403, 1330 cm 1. 1H NMR (500 MHz, CDCl3):
5.49 (s, 4H, CH2Ph); 7.30–7.40 (m, 10H, H–arom); 8.15 (s, 2H, H-8); 9.07 (s, 2H, H-2). 13C NMR (100.6 MHz, CDCl3): 47.52 (CH2Ph); 90.89 (C ); 127.89, 128.80 and 129.28 (CH–arom.); 134.83 and 135.09 (C-5 and C–i-arom); 140.40 (C-6); 145.83 (CH-8); 152.04 (C-4); 152.84 (C-2). EI HR MS, found: 442.1691; C26H18N8 [M] requires: 442.1654.
24.Review: Siemsen, P.; Livingston, R. C.; Diederich, F.
Angew. Chem. Int. Ed. Engl. 2000, 39, 2632.
25.Hay, A. S. J. Org. Chem. 1962, 27, 3320.
1058 |
M. Hocek, I. Votruba / Bioorg. Med. Chem. Lett. 12 (2002) 1055–1058 |
26. Typical procedure: A solution of CuCl (20 mg, 0.2 mmol), TMEDA (37 mL, 0.25 mmol) in DME (2 mL) was stirred at ambient temperature while a solution of 1a (244 mg, 1 mmol) in DME (8 mL) was added dropwise. The stirring of the mixture in air atmosphere was continued for 4 h and was allowed to stand overnight. Then the solvent was evaporated and the residue was chromatographed on silica gel (100 g, ethyl acetate–light petroleum 1:1) to give the 1,4-bis(9-benzylpurin-6-yl)butadiyne (7a) (137 mg, 59%); mp 215 C dec. (CH2Cl2/heptane); FAB MS, m/z (rel.%): 467 (8) [M+H], 91 (100). IR (CHCl3): n=2157, 1575, 1497,
1491, 1457, 1435 1404, 1330 cm 1. 1H NMR (400 MHz, CDCl3): 5.46 (s, 4H, CH2Ph); 7.26–7.38 (m, 10H, H-arom); 8.12 (s, 2H, H-8); 9.01 (s, 1H, H-2). 13C NMR (100 MHz, CDCl3): 47.55 (CH2Ph); 78.59 and 80.68 (C C); 127.96, 128.83 and 129.27 (CH–arom.); 134.62 and 135.52 (C–arom and C-5); 139.72 (C-6); 145.80 (CH-8); 151.98 (C-4); 152.81 (CH-2). FAB HR MS, found: 467.1700; C28H19N8 [M+H] requires: 467.1732. Anal. calcd for C28H18N8 (466.5): C, 72.10; H, 3.89; N, 24.02; found: C, 71.96; H, 3.82; N, 23.81.
27. For experimental details of the assays see ref 16a.
