
Supplement F2: The Chemistry of Amino, Nitroso, Nitro and Related Groups.
Edited by Saul Patai Copyright 1996 John Wiley & Sons, Ltd.
ISBN: 0-471-95171-4
CHAPTER 19 |
|
Rearrangement reactions |
|
involving the amino, nitro |
|
and nitroso groups |
|
D. LYN H. WILLIAMS |
|
Chemistry Department, University of Durham, Durham, U.K. |
|
Fax: (191)-386-1127; e-mail: D.L.H.WILLIAMS@DURHAM.AC.UK |
|
I. INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
857 |
II. REARRANGEMENT OF HYDRAZOBENZENES |
|
(THE BENZIDINE REARRANGEMENT) . . . . . . . . . . . . . . . . . . . . . |
858 |
III. REARRANGEMENT OF AZOXYBENZENES (THE WALLACH |
|
REARRANGEMENT) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
865 |
IV. REARRANGEMENT INVOLVING PHENYLHYDROXYLAMINES . . . |
867 |
A. The Bamberger Rearrangement . . . . . . . . . . . . . . . . . . . . . . . . . . |
867 |
B. Other Rearrangements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
871 |
V. REARRANGEMENT OF N-HALO COMPOUNDS . . . . . . . . . . . . . . |
873 |
VI. REARRANGEMENT INVOLVING NITRO GROUPS . . . . . . . . . . . . . |
876 |
A. The Nitramine Rearrangement . . . . . . . . . . . . . . . . . . . . . . . . . . . |
876 |
B. Rearrangement of Nitro Aromatics . . . . . . . . . . . . . . . . . . . . . . . . |
879 |
C. Other Rearrangements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
882 |
VII. REARRANGEMENT INVOLVING NITROSO GROUPS . . . . . . . . . . . |
883 |
A. The Fischer Hepp Rearrangement . . . . . . . . . . . . . . . . . . . . . . . . |
883 |
B. Other Rearrangements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
885 |
VIII. REFERENCES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
888 |
I. INTRODUCTION
Rearrangement reactions have greatly interested chemists for a long time (a) from the synthetic viewpoint as routes to new compounds and (b) from the mechanistic viewpoint, in order to discover how these reactions occur. This chapter is intended to update previous chapters in this series. No attempt has been made to be comprehensive in the treatment, and the effort has centred on new developments, particularly of understanding mechanisms, rather than on reporting additional examples of reaction types already known. Rearrangement reactions are constantly being reviewed; the most complete account is in
857
858 |
D. Lyn H. Williams |
that chapter devoted to rearrangements covering the literature each year in the Organic Reaction Mechanisms series1. A short account aimed at undergraduates has appeared within the Oxford Primer series2.
Two major new developments have occurred since the subject was last covered in this series and have been instrumental in being able to give definitive answers to mechanistic questions, which hitherto relied to some degree on speculation. The first is the ability to measure, with the necessary accuracy of measurement, heavy-atom kinetic isotope effects. Kinetic isotope effects (KIE) involving 1H and 2H isotopes have always played an important part in establishing reaction mechanisms, and such KIE values have been easy to measure experimentally, because of their relatively large magnitude. Isotope effects for bond breaking and making involving C, N and O isotopes particularly are now measurable and have been applied to the study of rearrangement reactions, particularly by Shine and his group at Texas Tech University. These studies have enabled chemists to decide in a rearrangement reaction whether bond breaking and bond making are synchronous processes, or whether the former precedes the latter resulting in at least a two-stage process with intermediate formation. The other technique is the application of the CIDNP effect, particularly by Ridd and coworkers. Enhancement of NMR signals is frequently an indication that radical pairs or radical ion-radical pairs are involved as intermediates. This ability to establish the nature of the intermediates clearly is a major tool in reaction mechanism studies.
II. REARRANGEMENT OF HYDRAZOBENZENES (THE BENZIDINE
REARRANGEMENT)
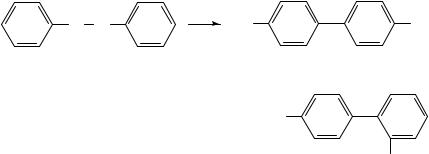
This is one of the most well-known (and unusual) rearrangement reactions involving amino groups and has probably received the most attention mechanistically speaking of all rearrangement reactions. It is set out formally in Scheme 1. The reaction is most wellknown under conditions of acid catalysis, although thermal and photochemical reaction pathways are also known. In acid solution hydrazobenzene 1 (more properly known as N,N0 -diphenylhydrazine) rearranges to give 4,40 -diaminobiphenyl (2) usually in about 70% yield together with 2,40 -diaminobiphenyl 3 (ca 30%). The common name for 2 is benzidine (after which the rearrangement is generally known) and for 3 diphenyline. Three other products have been detected usually with substituted hydrazobenzenes, and often in low yield. These are the 2,20 -diaminobiaryl 4, much more common from the reaction of hydrazonaphthalenes and the two arylaminoanilines 5 and 6 generally referred to as the ortho- and para-semidines, respectively. Often, products of disproportionation ArNH2 and
NH NH |
H |
+ |
NH2 |
|
H2 N |
||
(1) |
|
(2) |
(70%) |
+ H2 N
NH2
(3) (30%)
SCHEME 1

19. Rearrangement reactions involving the amino, nitro and nitroso groups 859
|
|
|
|
NH |
R |
|
R′ |
R |
R′ |
NH2 |
NH2 |
|
|
NH2 |
|
(4) |
|
|
(5) |
|
H2 N |
|
NH |
|
|
R |
|
|
R′ |
|
|
|
(6) |
|
ArNDNAr are also formed. Reaction occurs for a whole range of R and R0 substituents, often leading to a large spread of products if both 4- and 40 -positions are substituted. In some cases (for R D SO3H, CO2H) the substituent group R can be displaced. The reaction also can occur when there are N-substituents. Interestingly there is a recent report3 that when the rearrangement of 1 takes place in the presence of a rhodium(I) catalyst, the ortho-semidine product 5 is formed exclusively. Clearly the stereochemistry of the system when the catalyst is bound must be ideally set up for this product formation.
The rearrangement was reviewed in this series in 19684 and 19755 and there have been many other important reviews6 8. Interestingly from a historical viewpoint is the account of the very early history of the benzidine rearrangement which includes the possible contributions from the chemist/musician Borodin9. Reaction is clearly intramolecular as shown by a range of experiments where no cross-over products were observed, and also by isotopic labelling experiments. Kinetic measurements showed that reaction was first-order in the hydrazo compound and both a firstand second-order dependence upon the acidity occurred depending on the reactant structure and the acidity of the medium. Various mechanisms have been postulated, but two emerged as the most likely contenders during the 1960s and 1970s. These were the -complex mechanism put forward and argued by Dewar10, and later the Polar Transition State mechanism advocated by Banthorpe,
Hughes and Ingold11. Both mechanisms eventually attempted to incorporate reaction path-
+
ways via the monoprotonated reactant ArNHNH2Ar and also via the diprotonated reactant
+ +
ArNH2NH2Ar.
A major breakthrough in the mechanistic investigation of this and many other rearrangements occurred when it became possible to measure, within the accuracy required, heavy-atom kinetic isotope effects, i.e. those involving bond breaking and making of bonds to elements other than hydrogen. In particular for the benzidine rearrangement, the ability to measure these KIE for 14N 14N/15N 15N for bond breaking, and the carbon isotope effects (for 12C/13C and 12C/14C) for bond making, has meant that for the first time definitive reaction pathways have been firmly established. This has been due to the pioneering work of Shine and his group, begun in 1976. Heavy atom KIE are necessarily small (typically 1% 5%) so it is not possible to obtain meaningful values by kinetic measurements of the isotopically substituted materials. All of the data were obtained by competition methods12, using isotope-ratio mass spectrometry, whole-molecule-ion mass spectrometry and scintillation counting procedures. Details of the methods are given in the literature13 15. An account of the results obtained up to 1989 has been given by Shine16.
860 |
D. Lyn H. Williams |
This also includes an excellent summary of earlier work, particularly on the position at various times of the -complex theory.
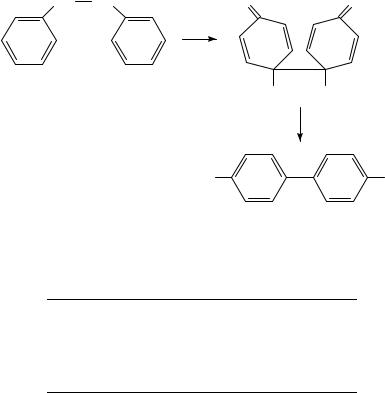
The results obtained by the Shine group17 for the reaction of hydrazobenzene itself are given in Table 1. The main point of note is that for the bond-making process the KIE values are different for the formation of 2 and 3. There is a KIE of the expected magnitude on N,N bond breaking (although they are not the same) for both 2 and 3 formation. However, for C C bond formation (using both the 13C and 14C isotopes) 2 formation shows the expected KIE for a rate-limiting process whereas for 3 formation there is no measurable KIE. The clear conclusion is that rearrangement of 1 to 2 is a concerted process whereas that for 3 formation is not, the rate-limiting step being N N bond fission. There is no deuterium KIE for C H bond breaking from the 4- and 40 - positions (other than a small inverse secondary effect), so this final proton loss must occur after the rate-limiting step. There is, as expected, no KIE for the 2,20 ,6,60 -13C labelled material. Rearrangement to give 2 is clearly a concerted process and can be classified as an allowed [5,5]-sigmatropic rearrangement, to form the quinonoid intermediate from which rapid proton transfer to the solvent occurs to give the final product (Scheme 2). This idea was in fact suggested as a possibility some twelve years earlier by Schmid18 following elegant isotope work on the Claisen rearrangement. It is also in effect a part of the Polar Transition State mechanism.
+ |
+ |
+ |
+ |
NH2 |
NH2 |
NH2 |
NH2 |
|
|
Slow |
|
|
|
H |
H |
|
|
|
Fast |
|
|
H2 N |
NH2 |
|
|
|
(2) |
|
|
SCHEME 2 |
|
TABLE 1. Values of the KIE for the acid catalysed rearrangement of 1 to give 2 and 3
Label |
Formation of 2 |
Formation of 3 |
|
|
|
|
|
15N,15N0 |
1.0222 |
1.0633 |
|
4,40 |
-13C |
1.0209 |
1.0006 |
4-14C |
1.0284 |
1.0011 |
|
2,20 |
,6,60 -13C |
0.9945 |
0.9953 |
4,40 |
-2H |
|
0.962a |
a Measured for the disappearance of 1.
19. Rearrangement reactions involving the amino, nitro and nitroso groups 861
Rearrangement to the diphenyline product 3, formally a forbidden [3,5] shift, must take place by a different mechanism in parallel to 2 formation. Previous mechanistic suggestions have attempted to explain the formation of both products within the same mechanistic framework. It is now apparent that 3 is formed by rate-limiting N-N bond fission to give an intermediate from which the product is formed. The nature of this intermediate is not yet known, but it has been suggested16 that it could be a -complex.
The reaction of hydrazobenzene (1) refers to reaction via the doubly protonated form. The mechanism for the rearrangement via a mono protonated form was examined17 using 2,20 -dimethoxyhydrazobenezene (7). Again the KIE results for formation of the benzidine derivative 8 show that reaction is also concerted. It appears that there is no major difference between the oneand two-proton reactions.
OMe |
MeO |
|
MeO |
OMe |
NH |
NH |
H |
+ |
NH2 |
|
H2 N |
|||
(7) |
|
|
(8) |
|
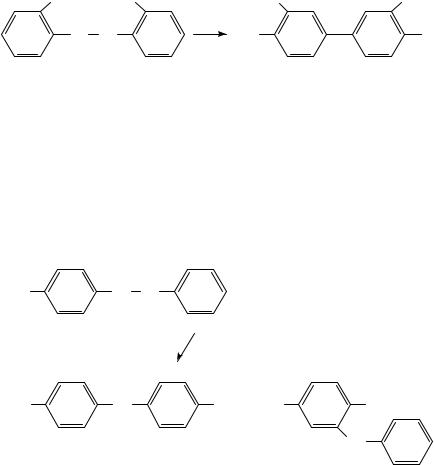
Reactions leading to the semidine products have also been examined by the heavy-atom KIE method19. The reaction of 9 gives both 10 and 11. As expected, a KIE on N N bond breaking was found for both products. For formation of 10 there was also a carbon KIE indicative of concertedness. The para-semidine rearrangement is then a [1,5]-sigmatropic shift. It was not possible to obtain the corresponding values for the formation of 11 (the ortho-semidine rearrangement). In an attempt to examine ortho-semidine formation the reaction of the 4,40 -dichlorohydrazobenzene (12) was studied20. This reaction gives the ortho- (13) and the para- (14) semidines as well as a significant amount of the products of disproportionation 15 and 16 (Scheme 3). The formation of 14 is accompanied by loss of chlorine. There was no carbon KIE for bond formation for the formation of both semidine products 13 and 14 but the expected nitrogen KIE for both, so that in this case neither rearrangement product is formed in a concerted process.
MeO |
NH NH |
(9)
H+
MeO |
NH |
NH2 + MeO |
NH2 |
|
|
|
NH |
|
(10) |
|
(11) |
Details of the mechanism of the ortho-benzidine rearrangement were examined using the two hydrazonaphthalene derivatives 17 and 1817,21. Both showed nitrogen and carbon

862 |
|
|
D. Lyn H. Williams |
|
|
|
|
|
Cl |
NH2 |
|
|
|
|
|
NH |
Cl |
Cl |
NH |
NH |
Cl |
(13) |
|
|
(12) |
|
|
|
|
|
|
|
Cl |
NH |
NH2 |
|
|
|
|
(14) |
|
Cl |
N |
N |
NH2 |
+ 2 NH2 |
Cl |
|
(15) |
|
|
(16) |
|
|
|
|
SCHEME 3 |
|
|
NH2
NH NH |
NH2 |
|
(17)
NH NH
NH2
(18) |
NH2 |
19. Rearrangement reactions involving the amino, nitro and nitroso groups 863
isotope effects showing the reactions to be concerted and can be regarded as [3,3]- sigmatropic shifts.
One other set of experimental results has been reported giving the results of heavyatom KIE experiments in the benzidine rearrangement22. A feature of percyclic reactions (including sigmatropic rearrangements) is that the motion of all the atoms involved are coupled. It follows that kinetic isotope effects should be found for atoms not directly involved in bond breaking or making, so that conventional secondary effects should be much greater than those normally encountered for the reactions. This has been tested in the reaction of hydrazobenzene itself using both carbon isotopes at the 1- and 10 -positions using 1-14C and 1,10 -13C2 labelled reactants. As predicted, there was a small but significant KIE for the reaction leading to the benzidine product (2) but no measurable KIE on the reaction leading to diphenylene (3). This is entirely consistent with the earlier findings and interpretation that 2 is formed in a concerted process whereas 3 is not but requires the formation of an intermediate. This paper22 also reports the results of the repetition of earlier experiments using 1 for 4,40 -13C2, 4-14C and 15N, 15N0 labelled compounds. The results are somewhat different from those reported earlier, but it is argued that the more recent results are likely to be the more reliable given the better scintillation counting and mass spectrometric facilities available.
As mentioned earlier, products of disproportionation often accompany the rearrangement products. This reaction is also acid-catalysed and it is a reasonable assumption that reaction proceeds via the protonated species. Experiments with the 4,40 - diiodohydrazobenzene (19) showed that there were significant nitrogen and para-carbon kinetic isotope effects23. This implies that disproportionation must take place after C C bonding has occurred, i.e. that the intermediate must be the quinonoid form 20 (and cannot, for example, be a -complex), which is then believed to react with another reactant molecule to give the disproportionation products (Scheme 4).
As a result of these heavy-atom KIE experiments the principal features of the benzidine rearrangements have now been firmly established. The two main products arise from two parallel reactions one of which is concerted and the other is not. Other concerted processes have been identified and all of the concerted processes can be readily classified in the terminology of sigmatropic rearrangements within the general class of percyclic reactions.
The benzidine rearrangements can also be brought about thermally, but very few mechanistic studies have been carried out. One set of heavy-atom KIE measurements has been made in the reaction of 2,20 -hydrazonaphthalene (18)21. Substantial nitrogen (1.0611 for the [15N, 15N0 ]) and carbon (1.0182 for the [1,10 -13C2]) KIE values were obtained showing that, just as for the acid catalysed reaction, this is a [3,3]-sigmatropic rearrangement, this time presumably of the non-protonated reactant.
There continue to be a few examples reported where rearrangement has been used synthetically to develop new products sometimes important in the industrial world. Monomers for polyamides and polyimides (which are used for making moisture sensitive films, fibres and mouldings) have been synthesized24 by the reduction of a nitro compound, followed by a benzidine rearrangement of the resulting hydrazobenzene derivative as outlined in Scheme 5.
Similarly, a number of 2-(2-arylhydrazino) tropones undergo the benzidine rearrangement when treated with HCl in EtOH to give 2-amino-5-(4-aminoaryl)tropones, which can be hydrolysed to the corresponding 5-aryl-tropolones. This is a useful route to a synthesis for open B ring colchine analogues25.
Quinamine Rearrangement
4-(Arylamino)-cyclohexadienones (21) rearrange in acid solution (often in alcohol or acetic acid solvents) to give 4-(aryloxy)-anilines (22) (Scheme 6). In some ways this

864 |
D. Lyn H. Williams |
|
I |
NH NH |
I |
(19)2H+
+ |
I |
+ |
|
||
H2 N |
|
NH2 |
|
I |
|
|
(20) |
|
20 + I |
NH NH |
I |
+
NH3 |
|
|
|
2 |
+ I |
N N |
I |
I |
|
|
|
|
|
SCHEME 4 |
|
CF3 O |
|
OCF3 |
|
NH |
NH |
|
|
|
|
H+ |
|
|
Reduction |
CF3 O |
OCF3 |
|
OCF3 |
|
|
|
|
H2 N |
NH2 |
|
|
|
Condensation |
|
NO2 |
Polyamide |
|
|
|
||
SCHEME 5
|
19. Rearrangement reactions involving the amino, nitro and nitroso groups |
865 |
|||||||||||
|
NH |
|
O |
|
NH2 |
|
|
O |
Me |
||||
|
|
|
|
||||||||||
R |
Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R1 |
|
|
R |
|
|
R1 |
|
||||
|
(21) |
|
|
|
|
|
|
(22) |
|
|
|
|
|
|
|
|
|
SCHEME 6 |
|
|
|
|
|
|
|
|
|
reaction bears a formal resemblance to the benzidine rearrangement. Kinetic measurements have quantified acid catalysis and the lack of crossover products has shown the reaction to be intramolecular26. There remained the question of whether rearrangement was concerted or not and this has been addressed by Boduszek and Shine27, who measured the KIE for the [18O], [15N] and [4-14C] (at the para position of the aniline ring) isotopes. The values obtained, k16O/k18O 1.0399, k14N/k15N 1.0089 and k12C/k14C 1.0501, confirmed that the rearrangement is concerted and is a [5,5]-sigmatropic shift. Other minor products are formed resulting from another concerted [3,3]-sigmatropic change.
III. REARRANGEMENT OF AZOXYBENZENES (THE WALLACH
REARRANGEMENT)
In some way formally similar to the benzidine rearrangement is the Wallach rearrangement of azoxybenzene 23 to give 4-hydroxyazobenzene 24 in concentrated (typically 95%) H2SO4. The 2-hydroxy isomer is sometimes formed in low yield with some substituted azoxybenzenes, and it is the main product in the photochemically induced reaction. Much of what is known about the reaction has been covered in earlier review articles28 30. This contribution will report work published since 1981.
+ O− |
H2 SO4 |
|
N |
N |
|
N |
N |
OH |
(23) |
(24) |
|
With 4,40 -substituted azoxybenzenes a range of different products has been reported. For example, with electron-withdrawing groups such as NO2, COCH3, CO2H reaction gives the ‘normal’ 2-hydroxy isomer (25) plus the 40 -hydroxy product 26 believed to be formed by ipso-attack by water from the solvent and expulsion of the nitro group31. Another example of ipso-attack at the 40 -position, this time followed by rearrangement, occurs in the reaction of 4,40 -dialkylazoxybenzene when the products are 27 and 28, believed to be
HO
O2 N |
N |
N |
NO2 |
(25)
866 |
D. Lyn H. Williams |
|
O2 N |
N |
|
|
N |
OH |
(26)
R |
N |
R |
N |
|
|
N |
R |
N |
OH |
|
|
OH |
|
R |
|
|
|
|
|
|
(27) |
|
(28) |
|
R |
N + |
R |
|
|
|
N |
|
|
|
|
|
OH |
|
|
R |
N |
|
R |
|
N |
OH
+
(29)
generated from the intermediate 2932. When the 4- and 40 -substituents are halogens, then the major product is that of reduction, the 4,40 -dihaloazobenzene33.
Interconversion of the isomers 30 and 31 occurs in the reactant during the rearrangement, involving an intermediate which is suggested as the bridged ion 32. This change occurs for X D Me and NO2 but strangely not when X D Br34. A remarkable difference in reactivity exists between two such isomers in phenylazoxypyridine35. The ˛ isomer
(33)is virtually inert towards rearrangement in 95 100% H2SO4 whilst the ˇ isomer
(34)reacts ‘normally’ to give 4-(40 -hydroxyphenylazo) pyridine-N-oxide. The reactivity difference is so great, that from mixtures of 33 and 34 pure samples of 33 can be obtained when all 34 has rearranged.
The Wallach rearrangement has been reported for perfluoro derivatives36. Reactions are much slower, as expected, and in sulphuric acid the octafluoro compound 35 gave no rearrangement product, but in chlorosulphuric acid rearrangement did occur to give the 4-chlorosulphonate ester.
Mechanistically speaking there have been no recent advances. What is known is that, at least for 4-OH formation, the reaction is intermolecular, requires two proton transfers at some stage and that a symmetrical intermediate is involved (often described as
