

IV.6 Arene Substitution via
Addition–Elimination
IV.6.1 Arene Analogs of the Heck Reaction
KEISUKE SUZUKI and KEN OHMORI
A. INTRODUCTION
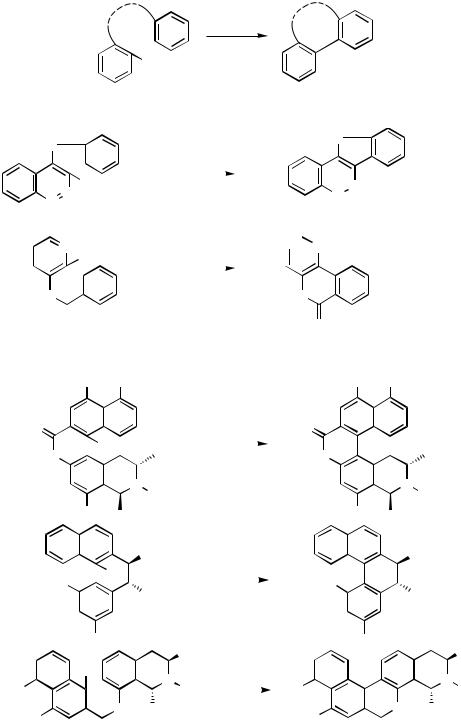
This section describes the formal arene analogs of the Heck reaction (Scheme 1), which offers one of the simplest ways of synthesizing biaryl compounds from the corresponding arenes and aryl halides (X Br, I) or sulfonates (X OSO2R) under palladium catalysis. The reaction is typically conducted in polar solvents (DMF or DMA) at relatively high temperatures (120–170 °C) with Pd(OAc)2 or Pd(PPh3)2Cl2 as the catalyst in the presence of a base. Most of the existing examples have been restricted to intramolecular reactions with a temporary or nontemporary tether between the arene and the aryl halide, yet intermolecular couplings of this type, at least in a formal sense, have also been observed. These examples will be discussed in the last part of this section (see also Sect. IV.6.2).
B. INTRAMOLECULAR ARYLATION REACTIONS OF ARYL HALIDES
An early example of this arene coupling was reported by Ames and Bull,[1] who noted the formation of benzofuro[3,2-c]cinnoline in the reaction of 3-bromo-4-phenoxycinnoline with ethyl acrylate in the presence of a catalytic amount of palladium(II) acetate (Scheme 2, Eq. 1). Subsequent studies showed that ethyl acrylate is not necessary, and the arylation reaction was achieved by using 0.1 equiv of palladium acetate, 0.2 equiv of triphenylphosphine, and 3 equiv of sodium acetate in DMA at 170 °C (Scheme 2, Eq. 2).[2]
Subsequent extensive studies by Bringmann and co-workers proved the potential of this intramolecular aryl–aryl coupling for the synthesis of various natural products containing highly substituted biaryl substructures (Scheme 3); two aromatic residues are first linked and then cyclized by this type of coupling in a regioand stereoselective manner.[3]–[7] The tether, irrespective of being temporary or permanent, secures not only high yields but also the regioselectivity of the C—C bond formation. Notably, biaryl bond formation could be realized for highly hindered substrates, for which the use of the Herrmann–Beller catalyst I [8],[9] is recommended.
Handbook of Organopalladium Chemistry for Organic Synthesis, Edited by Ei-ichi Negishi ISBN 0-471-31506-0 © 2002 John Wiley & Sons, Inc.
1471
1472 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
cat. Pd(0)
–HX
X
Scheme 1
|
X |
|
|
Pd(OAc)2 |
||||
|
|
|
|
|
|
|
|
ethyl acrylate |
|
|
|
|
|
|
|
|
Et3N, MeCN |
|
|
|
Br |
150 °C, 5 h |
||||
|
|
N |
|
|||||
|
N |
|
||||||
|
N |
Pd(OAc)2 |
||||||
|
||||||||
|
|
|
Cl |
NaOAc/DMA |
||||
|
|
|
|
|
|
|
|
|
HN |
|
|
|
|
170 °C, 20 h |
|||
|
|
|
|
|||||
|
|
42% |
||||||
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O
X
(1)
 N N
N N
X = O (19% + 33% S.M. recovery) X = NH (55%)

 N
N
(2)
HN
O
|
|
|
|
|
|
|
|
|
|
|
Scheme 2 |
|
|
|
|
|
|
|
|
|
|
||
|
MeO MeO |
|
|
|
|
|
|
|
MeO MeO |
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
PdCl2(PPh3)2 |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
O |
|
|
O |
|
|
||||||||||||||||||
|
|
|
DMA, 95 °C |
|
|
||||||||||||||||||
|
|
|
|
Br |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
82% |
|
|
|
O |
|
|
|||||||||||
|
O |
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
N |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
Bn |
|
|
|
|
|
|
|
|
|
|
|
|
|
Bn |
|
|
MeO |
|
|
|
|
|
|
|
MeO |
|
|
||||||||||||
|
|
|
|
|
|
|
|
OAc |
PdCl2(PPh3)2 |
|
|
|
|
|
|
|
|
|
OAc |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
DMA |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
Br |
|
|
120−140 °C |
|
|
|
|
|
|
|
|
|
|
|
||
|
MeO |
OAc |
90% |
|
|
MeO |
|
OAc |
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MeO |
Me |
|
|
|
|
|
|
MeO |
|
Me |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
Br |
|
|
|
PdCl2(PPh3)2 |
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
N |
|
DMA, 130 °C |
i-PrO |
|
|
|
|
|
N |
||||||
i-PrO |
|
|
|
|
|
Bn |
|
|
|
|
|
|
|
|
Bn |
||||||||
|
|
|
|
|
75% |
|
|
|
|
O |
|
||||||||||||
|
|
|
|
|
|
|
O |
Me |
|
|
|
|
|
|
|
|
|
|
Me |
||||
MeO |
|
|
|
|
|
|
|
MeO |
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
Scheme 3 |
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
IV.6.1 |
ARENE ANALOGS OF THE HECK REACTION |
1473 |
|||||||
|
|
O |
I, NaOAc, |
|
|
|
|
Ar |
Ar |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
O |
P o |
o |
|
|
|
||||
|
|
Br |
DMA 160 °C |
|
|
|
|
|
Pd |
Pd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
t-Bu |
|
O |
81% |
|
t-Bu |
|
|
O |
|
|
|
|
|
|
|
|
o |
o |
P |
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
Ar = o-tolyl |
Ar |
Ar |
|
|||
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||
I
t-Bu |
MeO |
|
t-Bu |
||
|
|
|
|
|
|
O O |
|
|
|
|
|
|
Br |
|
|
PdCl2(PPh3)2 |
|
|
|
||||
|
|
|
|
NaOAc, DMA |
|
|
|
|
|
|
|
|
|
Me |
|
130 °C |
|
|
|
|
|
|
|
|
|
OR′ |
|
|
|
OR
MeO
O O
O
Me
OR′
OR
R, R′ = Me 39%, 41% of S.M. recovered R, R′ = Ph2C 87%
Scheme 3 (Continued )
The last example in Scheme 3 shows that certain precautions may have to be taken depending on the substrate structure. The poor result for the substrate with an -methoxy group was ascribed to the C—H insertion reaction of the intermediary arylpalladium species at the neighboring OMe group, which is related to the observation by Dyker.[10] By contrast, by employing a protecting group without an -hydrogen at the vicinal alkoxy group, an excellent yield of the aryl –aryl coupling product was obtained. This example at the same time shows the coupling reaction is rather tolerant to groups with high steric demand.
This coupling reaction has been applied to the synthesis of a number of other natural products including the gilvocarcins and the pradimicins (Scheme 4).[11]–[13]
|
|
BnO MeO |
|
|
|
|
|
|
|
|
BnO MeO |
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
PdCl2(PPh3)2 |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
NaOAc, DMA |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
Br |
130 °C, 2 h |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
O |
|
|
|
|
40% |
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|||||
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|||||||||
|
|
BnO MeO |
|
|
|
|
|
BnO MeO |
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
OMe |
PdCl2(PPh3)2 |
|
|
|
|
|
|
|
|
|
|
OMe |
|||
BnO |
|
|
|
|
|
|
I |
BnO |
|
|
|
|
|
|
|
|
|
|
|||||
H |
|
|
|
|
|
|
|
NaOAc, DMA |
H |
|
|
|
|
|
|
||||||||
Me |
|
|
|
|
|
|
|
Me |
|
|
|
|
|
|
|||||||||
|
O |
|
|
O |
|
|
|
|
125 °C, 5 h |
|
O |
|
O |
|
|
||||||||
|
BnO |
|
|
|
|
|
Me |
90% |
|
|
|
BnO |
|
|
Me |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
OBn |
|
|
|
|
O |
|
|
|
|
OBn |
|
|
O |
|||||||||
|
|
|
|
|
CO2Me |
|
|
|
|
|
|
|
CO2Me |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
O |
|
|
O |
|
|
|
|
|
|
|
|
|
O |
O |
|||||||
|
|
|
|
|
|
|
|
|
|
Pd(OAc)2, PPh3 |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
NaOPiv, DMA |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MeO |
|
|
|
|
I |
|
|
|
|
110 °C, 1.5 h |
MeO |
|
|
|
|
|
|
||||||
|
|
|
|
OH OMOM |
86% |
|
|
|
|
OH OMOM |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
Cl |
|
|
|
|
OMe |
|
|
|
|
|
|
|
|
Cl |
OMe |
||||||||
Scheme 4
1474 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
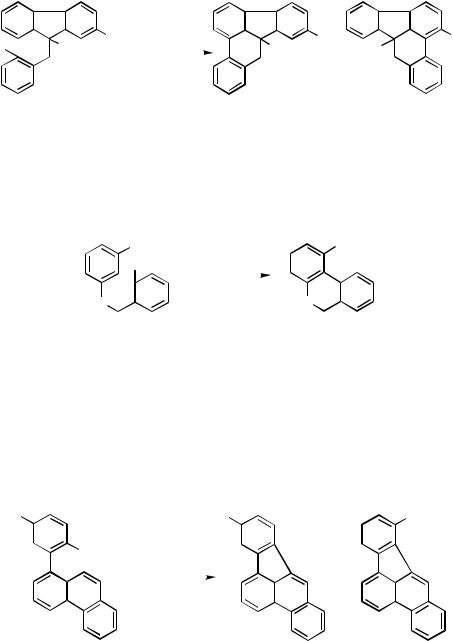
Echavarren and co-workers exploited this intramolecular arylation reaction for the synthesis of condensed polyaromatics.[14] The reaction of the aryl bromide derived from 9-methyl-2-nitrofluorene and -bromobenzyl bromide afforded two regioisomeric cyclized products (Scheme 5). It is noteworthy that the latter product arises from the coupling of the electron-deficient nitro-substituted arene moiety.
|
|
NO2 |
Pd(OAc)2, K2CO3 |
|
NO2 |
|
|
|
NO2 |
|
|
|
|
|
|
|
|||||
|
|
BnMe3NBr, DMF |
|
|
Me |
|||||
Br |
Me |
|
|
Me |
+ |
|
||||
100 °C, 24 h |
|
|||||||||
|
|
|
|
|
|
|
|
|
||
62%
2 : 1
Scheme 5
Rawal and co-workers reported that the reaction of phenolic substrates could be facilitated by generating the corresponding phenolate. The Herrmann–Beller catalyst I (vide supra) was particularly effective. Upon treatment with Cs2CO3 (3 equiv) and I (5 mol %) in DMA at 80 °C, the aryl iodide (Scheme 6) underwent smooth cyclization, thereby giving predominantly the “ortho-substituted” product in 97% yield.[15]
OH |
I |
OH |
||||
I |
|
|
|
|||
Cs2CO3, DMA |
|
|
|
|||
|
|
|
||||
O |
|
80 °C, 1 d |
|
O |
|
|
|
|
|
||||
|
97% |
|
|
|
||
|
|
|||||
Scheme 6
C. INTRAMOLECULAR ARYLATION REACTIONS OF ARYLSULFONATES
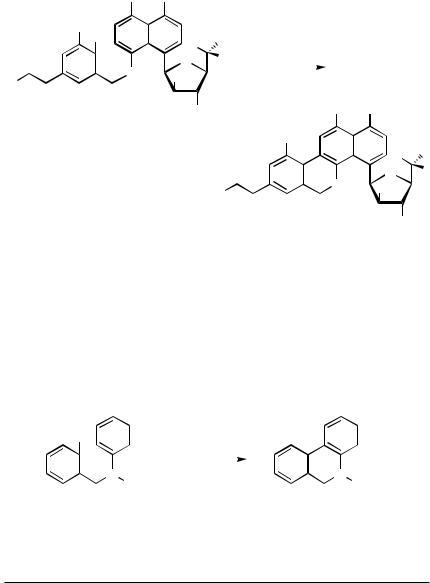
Instead of aryl halides as employed in the examples described so far, aryl triflates can be used as well, which significantly expands the scope of the reaction. The preparation of benzofluoranthenes, which proceeds in high yield when performed with 0.1 equiv of the palladium catalyst, 3 equiv of LiCl, and 1.2 equiv of DBU in DMA at 140 °C, exemplifies the situation (Scheme 7).[16] However, it is notable that two regioisomeric products were obtained, suggesting a mechanism with the possible involvement of radical intermediates.
F |
|
F |
|
|
F |
|||
|
|
|
|
|
|
|
|
|
|
|
OTf |
PdCl2(PPh3)2 |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
DBU, LiCl |
|
|
|
|||
|
|
|
DMF |
|
+ |
|
|
|
|
|
|
140 °C, 5 h |
|
|
|||
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
58%
1 : 1
Scheme 7
IV.6.1 ARENE ANALOGS OF THE HECK REACTION |
1475 |
Aside from the mechanistic aspects, this triflate protocol has significant potential in synthesis, as exemplified by the total synthesis of gilvocarcin V (Scheme 8).[17] The reaction of the triflate under the general conditions [PdCl2(PPh3)2, NaOAc, DMA, 125 °C, 5 h] led to the corresponding cyclized product in poor yield along with a side product, arising from the attack of an acetate at the ester carbonyl group with an enhanced electrophilicity by the -trifluorosulfonyloxy group. However, the yield could be improved significantly by employing sodium pivalate as a sterically hindered base.
|
|
MeO |
OBn |
|
|
|
|
|
|
|
|
|
MeO |
|
|
|
|
|
|
|
|
|
|||
|
OTf |
H |
OBn |
PdCl2(PPh3)2 |
|
|
|
|||||
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|||||
|
|
|
O |
O |
Me |
NaOPiv, DMA |
|
|
|
|||
|
|
|
|
|
|
|||||||
|
|
|
OBn |
|
125 °C, 5 h |
|
|
|
|
|||
MOMO |
|
|
|
|
||||||||
|
|
|
|
65% |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
OBn |
|
MeO |
|
OBn |
|
|||||
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
MeO |
|
H |
OBn |
|||
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
O |
Me |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
MOMO |
|
|
|
|
OBn |
|
||
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
O
OBn
Scheme 8
Recently, Harayama and co-workers reported an efficient protocol for the aryl triflate version of the coupling reaction (Scheme 9).[18] A highly reactive Pd catalyst, generated from Pd(OAc)2, dppp, and Bu3P as originally reported by Mandai, Matsumoto, and Tsuji, was used.[19] This protocol has been applied as the key step in a total synthesis of ravidomycin, in which the coupling proceeded nicely in the presence of a dimethylamino group (Scheme 10).[20]
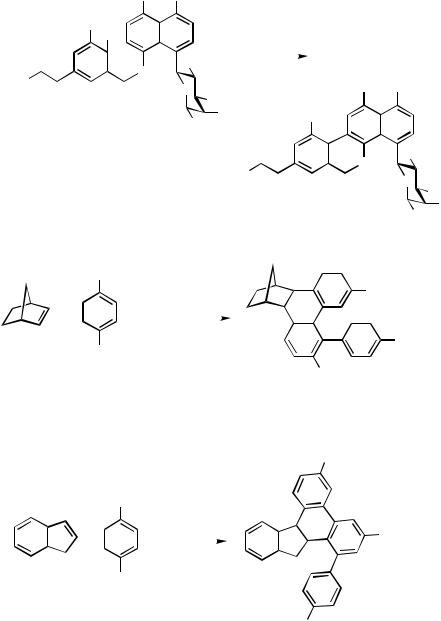
Judged from the outcome of the overall transformation, the domino 1:3 coupling of norbornene and aryl halides, first observed by de Meijere and co-workers,[21]–[23] is an
|
TfO |
|
|
conditions |
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
Ag2CO3 (2 equiv) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
DMF, reflux |
|
|
|
|
|
N |
|
||||
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
Et |
|
|
|
|
|
|
|
|
Et |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
O |
|
|||||||||
Pd(OAc)2 |
|
|
|
|
dppp |
P(n-Bu)3 |
Reaction Time |
Yield |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
30 mol % |
|
|
|
30 mol % |
3 equiv |
|
2 h |
71% |
||||||||
100 mol % |
|
|
|
100 mol % |
— |
|
190 h |
21%a |
||||||||
a24%, S.M. recovered.
Scheme 9
1476 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
intermolecular version of the arene analog of the Heck reaction presented in this section (for a more recent mechanistic discussion of the domino 1:3 coupling see Sect. IV.6.2). Under Jeffery conditions, norbornene and related bicyclic alkenes couple with aryl halides to form norbornane-annelated 4-aryl-9,10-dihydrophenanthrenes (Scheme 11, Eq. 1).
|
|
|
|
|
|
|
|
MeO |
|
OBn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
MeO |
|
|
|
|
|
|
|
|
|
|
Pd(OAc) |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
OTf |
|
|
|
|
|
|
|
dppp, P(n-Bu)3 |
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
OBn |
|
|
|
Ag2CO3, DMF |
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
130 °C, 15 min |
|
|
|
|||||||||||||
MOMO |
|
|
|
|
|
O |
70% |
|
|
|
|
|
|
MeO |
|
|
OBn |
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
|
|
|
|
|
|
O |
|
|
|
|
NMe2 |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
Me |
|
OBn |
MeO |
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MOMO |
|
|
|
|
|
|
|
O |
|
|
OBn |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
NMe 2 |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OBn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 10 |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
X |
|
|
|
|
Pd(OAc)2, K2CO3 |
|
|
|
|
|
|
|
R |
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
+ |
|
|
|
|
|
|
|
|
Bu4NBr, DMF |
|
|
|
|
|
|
|
|
|
|
|
(1) |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
65−80 °C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
X = I, Br |
|
|
|
|
X |
|
R |
|
|
Yield (%) |
|
|
R |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
I |
|
H |
83 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
I |
|
Me |
36 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
I |
|
OMe |
79 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
Br |
|
F |
48 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
I |
|
Cl |
38 |
|
|
|
|
|
R |
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
Br |
|
CN |
17 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
+ |
|
|
|
|
|
|
|
|
|
as above |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
(2) |
||
|
|
|
|
|
|
|
R |
|
X |
|
R |
Yield (%) |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
Br |
|
H |
35 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
I |
|
Me |
6 |
|
|
|
|
R |
|
|
|
||||||||||
Scheme 11
IV.6.1 ARENE ANALOGS OF THE HECK REACTION |
1477 |
Indene was found to react with aryl halides in an analogous way to yield 1:3 coupling products (Scheme 11, Eq. 2).[21],[24],[25]
REFERENCES
[1]D. E. Ames and D. Bull, Tetrahedron, 1982, 38, 383.
[2]D. E. Ames and A. Opalko, Tetrahedron, 1984, 40, 1919.
[3]G. Bringmann, J. R. Jansen, H. -P. Rink, Angew. Chem., Int. Ed. Engl, 1986, 25, 913.
[4]G. Bringmann, P. A. Keller, and K. Rölfing, Synlett, 1994, 423.
[5]G. Bringmann, W. Saeb, and M. Rübenacker, Tetrahedron, 1999, 55, 423.
[6]G. Bringmann, J. Hinrichs, J. Kraus, A. Wusik and T. Schulz, J. Org. Chem., 2000, 65, 2517.
[7]G. Bringmann, T. Pabst, P. Henschel, J. Kraus, K. Peters, E.-M. Peters, D. S. Rycroft, and
J.D. Connolly, J. Am. Chem. Soc., 2000, 122, 9127.
[8]W. A. Herrmann, C. Brossmer, K. Öfele, C.-P. Reisinger, T. Priermeier, M. Beller, and
H.Fischer, Angew. Chem. Int. Ed. Engl., 1995, 34, 1844.
[9]M. Beller, H. Fischer, W. A. Herrmann, K. Öfele, and C. Brossmer, Angew. Chem. Int. Ed. Engl., 1995, 34, 1848.
[10]G. Dyker, Angew. Chem., Int. Ed. Engl., 1992, 31, 1023.
[11]P. P. Deshpande and O. R. Martin, Tetrahedron Lett., 1990, 31, 6313.
[12]T. Matsumoto, T. Hosoya, and K. Suzuki, J. Am Chem. Soc., 1992, 114, 3568.
[13]M. Kitamura, K. Ohmori, T. Kawase, and K. Suzuki, Angew. Chem. Int. Ed. Engl., 1999, 38, 1229.
[14]J. J. González, N. García, B. Gómez-Lor, and A. M. Echavarren, J. Org. Chem., 1997, 62, 1286.
[15]D. D. Hennings, S. Iwasa, and V. H. Rawal, J. Org. Chem., 1997, 62, 2.
[16]J. E. Rice and Z.-W. Cai, J. Org. Chem., 1993, 58, 1415.
[17]T. Hosoya, E. Takashiro, T. Matsumoto, and K. Suzuki, J. Am Chem. Soc., 1994, 116, 1004.
[18]T. Harayama, T. Akiyama, and Y. Nakano, Chem. Pharm. Bull., 1997, 45, 1723.
[19]T. Mandai, T. Matsumoto, and J. Tsuji, Tetrahedron Lett., 1993, 34, 2513.
[20]S. Futagami, Y. Ohashi, K. Imura, T. Hosoya, K. Ohmori, T. Matsumoto, and K. Suzuki,
Tetrahedron Lett., 2000, 41, 1063.
[21]O. Reiser, M. Weber, and A. de Meijere, Angew. Chem. Int. Ed. Engl., 1989, 28, 1037.
[22]K. Albrecht, O. Reiser, M. Weber, and A. de Meijere, Tetrahedron Lett., 1992, 521.
[23]K. Albrecht, O. Reiser, M. Weber, B. Knieriem, and A. de Meijere, Tetrahedron, 1994, 50, 383.
[24]M. Weber, Dissertation, Universität Hamburg, 1992. The originally assigned structure (Ref. [21]) was revised according to two X-ray crystal structure analyses.
[25]A. de Meijere and S. Bräse, J. Organomet. Chem., 1999, 576, 88.
