

|
|
|
|
|
|
|
|
|
III.2.5 |
PALLADIUM-CATALYZED ARYL–ARYL COUPLING |
321 |
||||||
TABLE 5. (Continued ) |
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
ArMX PhY |
Cat. |
Ar−Ph |
|
|
|
|
|||
|
|
|
|
|
|
|
|
9: |
|
|
|
|
|||||
Entry |
|
|
|
|
|
|
Substrates |
MX |
Y |
Catalyst |
Yield (%) |
Reference |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
m-Mono |
|
|
|
|
|
|
|
||
8 |
|
|
|
|
|
|
MX |
Y |
SnBu3 |
Br |
Pd(PPh3)4 |
75 |
|
[38] |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Me |
|
|
|
|
|
|
|
|
|
|
||||||
9 |
|
|
|
|
|
|
MX |
Y |
B(OR)2 |
I |
Pd(PPh3)4 |
95 |
|
[66] |
|
||
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NH2 |
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
10 |
|
|
|
|
|
|
MX |
Y |
B(OH)2 |
Br |
Pd(PPh3)4 |
75 |
|
[59] |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Me3Si |
|
|
|
|
|
|
|
|
|
|
||||||
aArranged according to the following order of substitution pattern in aryl electrophiles: p-monosubstitued arylmetal m-monosubstituted arylmetal. In cases where the substituent pattern is the same, the substituents are arranged according to increasing order of priority determined by the Cahn–Ingold–Prelog rule.
TABLE 6 Pdor Ni-Catalyzed Aryl – Aryl Coupling Using 3,5- Substituted Arylmetals or Aryl Electrophilesa
|
|
|
|
|
ArMX Ar Y |
Cat. |
|
Ar – Ar |
|
|
||
|
|
|
|
|
9: |
|
|
|
||||
Entry |
|
|
Substrates |
|
MX |
Y |
Catalyst |
Yield (%) |
Reference |
|||
|
|
|
|
|
|
|
|
|
|
|
||
|
F |
|
|
|
|
|
|
|
|
|
||
1 |
|
|
MX |
Y |
|
B(OH)2 |
I |
PdCl2 |
65 |
[57] |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F |
|
|
|
|
|
|
|
|
|
||
2 |
|
|
MX |
Y |
|
B(OH)2 |
Br |
Pd(OAc)2 |
92 |
[67] |
||
|
|
|
||||||||||
|
|
|
|
|
|
EtO |
|
B(OH)2 |
Cl |
Pd(dba)2 + Ligandb |
94 |
[28] |
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
P O |
|
|
|
|
|
|
|
|
|
|
|
|
OEt |
|
|
|
|
|
|
3 |
|
|
MX |
Y |
|
B(OH)2 |
Br |
Pd(OAc)2, PPh3 |
86 |
[68] |
||
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
Ot-Bu |
|
|
|
|
|
|
|
|
|
|
|
|
N |
t-Bu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
COOMe |
|
|
|
|
|
|
4 |
|
|
MX |
Y |
|
B(OH)2 |
Br |
Pd(OAc)2 |
66 |
[69] |
||
|
|
|
|
|
|
COOMe |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Br |
|
|
|
|
|
|
5 |
|
|
MX |
Y |
|
B(OH)2 |
Br |
Pd(PPh3)4 |
67 |
[70] |
||
|
|
|
|
|
|
Br |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
aAryl electrophiles are arranged according to increasing order of priority determined by the Cahn–Ingold–Prelog rule. bLigand
Cy P

 O
O
Cy O

322 |
III Pd-CATALYZED CROSS-COUPLING |
TABLE 7. Pdor Ni-Catalyzed Aryl–Aryl Coupling Using 3,4-Substituted Arylmetals or Aryl Electrophilesa
|
|
|
|
|
|
ArMX Ar Y |
|
Cat. |
|
Ar–Ar |
|
|
|
|||||||||
|
|
|
|
|
|
9: |
|
|
|
|||||||||||||
Entry |
|
|
|
|
|
|
Substrates |
|
|
|
|
|
|
|
|
|
MX |
Y |
Catalyst |
Yield (%) |
Reference |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
|
|
|
|
|
MX |
Y |
|
|
|
|
|
|
|
|
|
B(OH)2 |
Br |
Pd(PPh3)4 |
87 |
[71] |
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
2 |
|
|
|
|
|
MX |
Y |
|
|
|
NH2 |
|
|
|
B(OH)2 |
I |
Pd(PPh3)4 |
52 |
[72] |
|||
BuO |
|
|
|
|
|
MX |
|
|
|
|
|
|
|
|
|
|
|
B(OH)2 |
Br |
Pd(DIPHOS)2 |
95 |
[73] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
3 |
|
|
|
|
|
Y |
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MgBr |
OMe |
NiCl2(PPh3)2 |
99 |
[74] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
4 |
|
|
|
|
|
MX |
Y |
|
|
|
|
|
|
|
|
MgBr |
Br |
Ph2(dba)3 + IPrHClb |
98 |
[27] |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OMe |
B(OH)2 |
Br |
Pd(OAc)2 |
99 |
[75] |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
Et |
|
|
B(OH)2 |
Br |
Pd(PPh3)4 |
95 |
[76] |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MgBr |
Br |
NiCl2(dppp) |
75 |
[77] |
|||
5 |
|
|
|
|
|
MX |
Y |
|
|
|
N |
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Y |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6 |
|
|
|
|
|
MX |
Y |
|
|
|
CN |
|
|
|
SnBu3 |
I |
Pd2(dba)3. CHCl3 |
72 |
[23] |
|||
|
|
|
|
|
|
|
|
|
|
|
CN |
|
|
|
|
|
|
|
|
|||
7 |
|
|
|
|
|
MX |
Y |
|
|
|
OH |
|
|
MgBr |
Br |
Cl2Pd(dppf) |
87 |
[43] |
||||
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
COOH |
|
|
B(OEt)2 |
Br |
Pd(OAc)2 |
99 |
[78] |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
8 |
|
|
|
|
|
MX |
Y |
|
|
|
S |
|
|
|
ZnCl |
Br |
PdCl2, K2CO3, H2O |
100 |
[79] |
|||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B(OH)2 |
Br |
Pd(PPh3)4 |
98 |
[80] |
|||
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
9 |
|
|
|
|
|
MX |
Y |
|
|
|
OMe |
|
|
MgBr |
Br |
NiCl2(PPh3)2 |
99 |
[74] |
||||
|
|
|
|
|
|
|
|
|
|
|
OMe |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
aAryl electrophiles are arranged according to increasing order of priority determined by the Cahn–Ingold–Prelog rule. bIPr 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene.
C. SYNTHESIS OF BIARYLS CONTAINING ONE OR TWO ORTHO SUBSTITUENTS VIA PdOR Ni-CATALYZED ARYL–ARYL COUPLING
Some representative examples of the synthesis of biaryls containing just one ortho substituent are shown in Table 8. The product yields are generally in the range of 70–100%, and Mg, Zn, B, and Sn are commonly used. Thus, one ortho substituent does not generally exert significant steric effects detrimental to the desired cross-coupling. Somewhat surprisingly, even those substituents that can chelate metals, such as COOMe[81] and CN,[26] have been successfully employed. All in all, the synthesis of biaryls containing only one ortho substituent appears to be closely analogous to those cases discussed in the preceding subsection.

III.2.5 PALLADIUM-CATALYZED ARYL–ARYL COUPLING |
323 |
TABLE 8. Pdor Ni-Catalyzed Aryl–Aryl Coupling Providing Biaryls Containing One Ortho Substituenta
|
|
ArMX Ar′Y |
|
Cat. |
|
Ar−Ar′ |
|
|
||
|
|
9: |
|
|
|
|||||
Entry |
|
Substrates |
|
MX |
Y |
Catalyst |
Yield (%) |
Reference |
||
|
|
|
|
|
|
|
|
|
|
|
1 |
|
MX Y |
|
|
|
MgBr |
Cl |
Ni(triphos)ClPF6 |
53 |
[82] |
|
|
|||||||||
|
|
MgCl |
Cl |
Pd(dppf)Cl2 |
79 |
[83] |
||||
Cl
2
3
4
5
6
7
8
 MX Y
MX Y
Br
 MX Y
MX Y
NO2
 MX Y
MX Y
 Ac
Ac
Me
 MX Y
MX Y
 NO2
NO2
Me
 MX Y
MX Y
 COOMe
COOMe
|
MX |
Y |
|
|
|
Me |
|
|
|
CN |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|||
|
MX |
Y |
|
|
|
N |
|
|
|
|
|
N |
|
|
|
|
N |
|
Me |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|||
|
|
|
|
|
|||||
N N |
t-Bu |
|
|
|
|
N |
|||
|
N |
|
|
|
Me |
|
|
||
|
|
|
|
|
|
|
|
|
|
MgBr |
Br |
Pd(dppb)Cl2 |
75 |
[84] |
B(OH)2 |
Br Pd(PPh3)4/Na2CO3 |
98 |
[85] |
|
B(OH)2 |
OTf Pd(PPh3)4 |
98 |
[86] |
|
SnMe3 |
OTf Pd2dba3. CHCl3 |
93 |
[87] |
|
B(OH)2 |
Br |
Pd2dba3 |
98 |
[87] |
ZnCl |
Br |
PdCl2(PPh3)2/DIBAH |
70 |
[9] |
B(OH)2 |
I |
Pd(OAc)2, K2CO3, H2O |
98 |
[87] |
ZnBr |
Cl |
NiCl2(PPh3)2 |
75 |
[26] |
ZnCl |
Br |
PdCl2(PPh3)2 |
78 |
[88] |
9 |
|
MX |
Y |
|
COOEt |
ZnI |
Br |
Pd(PPh3)4 |
100 |
[81] |
|
|
COOMe |
|
|
|
|
|
|
|
|
MeO |
|
|
|
|
CN |
|
|
|
|
|
10 |
|
MX |
Y |
|
CN |
SnMe3 |
I |
Pd2dba3. CHCl3 |
75 |
[23] |
|
|
OMe |
|
|
|
|
|
|
|
|
(Continued )

324 |
III Pd-CATALYZED CROSS-COUPLING |
TABLE 8. (Continued ) |
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
ArMX Ar′Y |
|
|
Cat. |
Ar−Ar′ |
|
|
||
|
|
|
|
|
|
|
|
9: |
|
|
||||
Entry |
|
|
Substrates |
MX |
Y |
Catalyst |
Yield (%) |
Reference |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
11 |
|
|
MX |
Cl |
|
|
|
|
ZnCl |
|
Ni(acac)2, dppf |
86 |
[26] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CN |
|
|
|
|
|
|
|||
12 |
|
|
MX |
Br |
|
|
|
|
Sn(Bu)3 |
Pd(PPh3)4 |
95 |
[47] |
||
|
|
|
|
|
COOH |
|
|
|
|
|
|
|||
13 |
|
|
MX |
Cl |
|
|
|
Me |
MgBr |
|
Pd(dba)2 + Ligandb |
85 |
[84] |
|
|
|
|
|
|
Me |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
Cl |
|
|
|
|
|
14 |
|
|
MX |
I |
|
|
|
|
|
|
|
TBAF, (allylPdCl) 2/(t-Bu)3P |
85 |
[60] |
|
|
|
|
|
|
Si |
|
|||||||
15 |
|
|
MX |
I |
|
|
|
|
ZnBr |
|
Pd(dba)2, tfpc |
76 |
[89] |
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
O |
|
|
|
|
|
|
|||
|
|
|
|
|
S(O)2(CF2)3CF3 |
|
|
|
|
|
|
|||
16 |
|
|
MX |
Br |
|
|
|
|
MgBr |
|
Ni(acac)2 |
72 |
[90] |
|
|
|
|
|
|
|
|
|
OMe |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
17 |
|
|
MX |
Br |
|
|
|
Br |
ZnCl |
|
Pd(PPh3)4 |
76 |
[89] |
|
|
3 : 1 |
OMe |
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
||||
18 |
|
|
MX |
Cl |
|
|
|
|
MgCl |
|
Ni(acac)2, (i-PrO)3P |
82 |
[91] |
|
|
|
|
|
|
CN |
|
|
|
ZnCl2 |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
||||
aAryl electrophiles are arranged according to increasing order of priority determined by the Cahn–Ingold–Prelog rule. bLigand
Cy P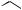

 O
O
Cy O
ctfp tris(o-furyl)phosphine.
III.2.5 PALLADIUM-CATALYZED ARYL–ARYL COUPLING |
325 |
The Pdor Ni-catalyzed reaction of 2,6-disubstituted arylmetals or aryl electrophiles appears to represent a point of deviation in that low product yields of 50% have been frequently reported, as indicated by the results shown in Table 9. Despite this unmistakable trend, the currently available data on this class of reactions are still limited and seemingly erratic or inconsistent. Consequently, they do not lend themselves to providing a reliable and useful set of generalizations. With the understanding that these cases share some critical features with the synthesis of biaryls containing three or four ortho substituents, it is recommended one consult the following subsection (Sect. D) for probing critical factors, such as metal countercations, leaving groups, and catalysts.
TABLE 9. Pdor Ni-Catalyzed Aryl – Aryl Coupling Providing Biaryls Containing Two Ortho Substituentsa
|
ArMX Ar′Y |
Cat. |
Ar −Ar′ |
|
|
9: |
|
||
|
|
|
|
|
Entry |
Substrates |
MX |
Catalyst |
Yield (%) Reference |
|
2,4-Di- |
|
|
|
1 |
|
|
|
|
MX |
Br |
|
|
|
|
|
2 |
|
|
|
|
MX |
Cl |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
||||
3 |
|
|
|
|
MX |
MsO |
|
|
|
CN |
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
OMe |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4 |
|
|
|
|
MX |
Br |
|
|
|
Br |
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
OMe |
1:1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
2,4,6-Tri- |
|
|
|
|
|
|
||||
5 |
|
|
|
|
MX |
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
Cl |
|
|
|
|
|
|
|
|
|
|
|
|
6 |
|
|
|
|
MX |
Br |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
7 |
|
|
|
|
MX |
Br |
|
|
|
CHO |
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||||
8 |
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
MX |
Cl |
|
|
|
|||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
Me |
|
|
|
|
|
|
|
|
|
|
|
|
9 |
|
|
|
|
MX |
Cl |
|
|
|
|
OMe |
|
|
|
|
|
|
|
|||||
B(OH)2 |
Pd(OAc)2, K3PO4 |
97 |
[92] |
B(OH)2 |
NiCl2(PPh2Et)2/ |
94 |
[29] |
|
zinc metal |
|
|
B(OH)2 |
NiCl2(dppf) |
56 |
[93] |
B(OH)2 |
Pd(PPh3)4, Cs2CO3 |
50 |
[94] |
B(OH)2 |
Pd(PPh3)4 |
92 |
[95] |
MgBr |
Ni complex |
41 |
[96] |
B(OH)2 |
Pd(PPh3)4 |
45 |
[40] |
B(OH)2 |
NiCl2(dppf) |
78 |
[25] |
MgBr |
Pd2(dba)3 . HCl |
95 |
[27] |
(Continued )

326 |
III Pd-CATALYZED CROSS-COUPLING |
|
|
|
|
|||||
TABLE 9. (Continued ) |
|
|
|
|
||||||
|
|
|
|
ArMX Ar′Y |
Cat. |
Ar −Ar′ |
|
|
||
|
|
|
|
9: |
|
|
||||
|
Entry |
|
|
Substrates |
MX |
Catalyst |
Yield (%) |
Reference |
||
|
|
2,4,5,6-Tetra- |
|
|
|
|
||||
|
|
|
|
|
|
CHO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10 |
|
|
MX Br |
SnBu3 |
Pd(PPh3)2Cl2 |
62 |
[97] |
|||
|
|
|
|
|
|
|
|
|
|
|
Br
11
12
13
14
15
16
17
18
Ph |
|
OMOM |
|
|
|
|
|
|
|
MX |
Br |
||||
|
|
||||||
|
|
|
|
|
|
N |
|
|
|
|
|
||||
Ph |
|
OMOM |
|
|
|
|
|
|
|
|
|
|
|
|
|
 MX I
MX I
 MX Cl
MX Cl
 MX TfO
MX TfO
 MX TfO
MX TfO
O 
N(i-Pr)2
|
|
|
MeOOC |
Me3Si |
|
|
I |
|
|
||
N |
|
MX |
|
Me3Si |
|
|
MeOOC |
|
|
|
ZnCl Ni(PPh3)Cl2 49 [98]
B(OH)2 |
Pd(OAc)2, PPh3 |
92 |
[92] |
MgBr |
Pd2(dba)3 . CHCl3 |
87 |
[27] |
MgBr |
Cl2Pd(dppp) |
94 |
[99] |
MgBr |
Ni(acac) |
47 |
[53] |
ZnCl |
Ni(PPh3)4 |
20 |
[100] |
|
|
MX |
Br |
|
|
SnBu3 |
Pd(PPh3)4 |
58 |
[38] |
Cl |
|
|
|
|
|
|
|
||
|
|
|
MeO |
|
O |
|
|
|
|
|
|
MX |
I |
|
O |
B(OH)2 |
Pd(OAc)2, K2CO3 |
100 |
[101] |
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
Ph |
|
|
|
|
|

|
|
|
|
|
|
|
III.2.5 |
PALLADIUM-CATALYZED ARYL–ARYL COUPLING |
327 |
||||||||||||||||
TABLE 9. (Continued ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
ArMX Ar′Y |
Cat. |
Ar −Ar′ |
|
|
|
|||||||||||||
|
|
|
|
|
|
|
9: |
|
|
|
|||||||||||||||
Entry |
|
|
|
|
|
|
Substrates |
|
|
|
|
|
|
|
|
|
|
|
MX |
Catalyst |
Yield (%) |
Reference |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OEt |
|
|
|
|
|
|
||
19 |
|
|
|
|
|
|
MX |
TfO |
B(OH)2 |
Pd(PPh3)4 |
96 |
|
[102] |
||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
H |
|
N |
Cl |
TBAF, |
|
|
[103] |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
20 |
|
|
|
|
|
|
MX |
|
|
I |
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
Si |
(allylPdCl)2/(t-Bu)3P 70 |
|
||||||||||||||
|
|
|
|
|
Me |
|
|
|
O2N |
|
|
|
|
|
|
||||||||||
21 |
|
|
|
|
|
|
MX |
|
Br |
MgBr |
NiCl2 |
49 |
|
[104] |
|||||||||||
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Me |
|
|
|
|
Cl |
|
|
|
|
|
|
|||||||||
22 |
|
|
|
|
|
|
MX |
|
Br |
SnBu3 |
PdBnCl(PPh3)2 |
95 |
|
[105] |
|||||||||||
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
NHC(O)Ot-Bu OHC |
|
|
|
|
|
|
||||||||||||||
23 |
|
|
|
|
|
|
MX |
|
Br |
SnBu3 |
Pd(PPh3)Cl2 |
74 |
|
[106] |
|||||||||||
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
NHC(O)Ot-Bu |
|
|
|
Cl |
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
24 |
|
|
|
|
|
|
MX |
|
Br |
|
|
O SnBu3 |
Pd(OAc)2 |
63 |
|
[107] |
|||||||||
|
|
|
|
|
|
|
|
||||||||||||||||||
|
MeO |
|
N |
|
C(O)Ot-Bu |
|
OHC |
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
MX |
|
|
|
|
|
|
|
|
|
|
SnMe3 |
Pd(PPh3)4, CuBr |
70 |
|
[103] |
|||
25 |
MeO |
|
|
|
|
Br |
|
||||||||||||||||||
|
|
|
|
|
NO2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
OHC |
|
|
|
|
|
|
||||||||||||
26 |
|
|
|
|
|
|
MX |
|
|
|
I |
MgBr |
Pd(PPh3)4 |
66 |
|
[108] |
|||||||||
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
OMe |
|
Me |
|
N |
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SiMe3 |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
aAryl electrophiles are arranged according to increasing order of priority determined by the Cahn–Ingold–Prelog rule.
Examples of the synthesis of biaryls containing two aryl groups, each of which contains one ortho substituent, seem to be rather rare. In the synthesis of magnolol and ( )-monoterpenylmagnolol shown in Scheme 3,[18] Zn was reported to be more satisfactory than Sn. The latter reaction was reported to be accompanied by double bond migration to significant extents.
328 |
III Pd-CATALYZED CROSS-COUPLING |
D. SYNTHESIS OF BIARYLS CONTAINING THREE
OR FOUR ORTHO SUBSTITUENTS
Biaryls containing three or four ortho substituents are discrete from the others in that they can exist as persistent atropisomers that are chiral. There are even a number of natural products containing such chiral biaryl moieties including michellamines A and B [109],[110] and vancomycin.[111] These compounds provide some of the ultimately challenging synthetic tasks. Specifically, two synthetic issues separate them from most of the other biaryls. One is dealing with three or four ortho substituents that exert strong steric hindrance to cross-coupling. Their electronic effects may also be significant in some cases. The other is controlling the absolute and relative stereochemistry of the atropisomers. Some noteworthy progress have been made recently along these lines. In recent synthesis of michellamines A and B,[17] Zn, B, and Sn were compared by using some model compounds. As the results shown in Scheme 2 indicate, both 1-iodo and 1- bromo derivatives of 2,4,6-tris(methoxy)benzenes are satisfactory cross-coupling partners in the Pd-catalyzed reaction with the 1-naphthylboronic acid derivative, whereas only the iodo derivative is satisfactory in the reaction of the 1-naphthylzinc derivative. Noteworthy is the complete failure observed with the 1-naphthyltin derivative.[17] Although these results represent just one study, the inferior reactivity of Sn as compared with Zn and/or B has been recorded in a growing number of more demanding cases of the Pd-catalyzed cross-coupling reactions, as discussed also in the following dozen or so sections in this part.
As indicated by the results shown in Tables 10 and 11, successful syntheses of biaryls containing three or four ortho substituents have indeed been reported by the use of Zn, B, and Mg. Although it is still premature to draw firm conclusions, areneboronic acids appear to lead to at least comparable and possibly even higher yields of the desired biaryls than arylmetals containing Zn or Mg, despite their significantly lower intrinsic reactivity. If true, this might stem from the facts that biaryls generally unassociated with delicate regioand/or stereochemical issues are thermally stable compounds and that areneboronic acids are much more thermally stable than the corresponding arylmetals containing Zn or Mg. These stability features must permit the formation of sterically hindered biaryls under forcing conditions with minimal complications arising from competitive side reactions including reagent decomposition.
In view of the significance of chiral biaryls, it may be predicted that many additional investigations along this line will be reported in the near future, and they should help provide a more definitive discussion of this topic.
The other important issue of enantioselective synthesis of chiral biaryls is discussed in Sect. III.2.16, and it is therefore not duplicated here.
E. SYNTHETIC APPLICATIONS OF THE PdOR Ni-CATALYZED
ARYL–ARYL COUPLING
Synthesis of biaryls via Pdor Ni-catalyzed aryl–aryl coupling has found many attractive applications in the synthesis of oligoand polyaryls and natural products containing biaryls. The former topic is discussed in Sect. III.2.17.2, and the latter is further supplemented in Table 1 of Sect. III.2.18.

|
|
|
|
III.2.5 PALLADIUM-CATALYZED ARYL–ARYL COUPLING |
329 |
|||||||
TABLE 10. Pdor Ni-Catalyzed Aryl–Aryl Coupling Providing Biaryls Containing Three |
|
|
||||||||||
Ortho Substituents |
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
ArMX Ar'Y |
Cat. |
Ar−Ar' |
|
|
|
|
||
|
|
|
|
9: |
|
|
|
|
||||
Entry |
|
Substrates |
MX |
Catalyst |
Yield (%) |
Reference |
||||||
|
|
|
|
|
|
|
|
|
|
|
||
|
|
Me |
|
OHC |
|
|
|
|
|
|
||
1 |
|
|
MX |
Br |
B(OH)2 |
Pd(PPh3)4 |
82 |
[112] |
|
|
||
|
|
|
|
|||||||||
|
|
B(OH)2 |
Pd(PPh3)4 |
80 |
[113] |
|
|
|||||
|
|
|
|
|
|
|
|
|
||||
Cr(CO)3
MeO
CHO
2 |
|
MX |
|
|
|
|
|
OMe |
3 |
|
MX |
|
||
|
|
OMe |
4 |
|
MX |
|
5 MOMO
 MX
MX
6 O
 MX
MX
7 |
O |
MX |
|
O |
CHO |
8
 MX
MX
9
 MX
MX
10
 MX
MX
I
MeO
TfO
MeO
MeO
TfO
MeO2C
Me
Y 
 OMe
OMe
MeO
Me Bn
 N
N


 Me
Me
Br
 OBn
OBn
BnO
HOH2C
Br
 OMe
OMe
Cr(CO)3
MeO OMe
Y
Cl
I
Cl
Br
|
B(OH)2 |
|
|
|
B(OH)2 |
|
|
|
B(OH)2 |
|
|
|
|
|
|
|
ZnCl |
|
Y = I |
|
B(OH)2 |
Y = I |
|
|
SnBu3 |
|
Y = I |
|
ZnCl |
|
Y = Br |
|
B(OH)2 |
Y = Br |
|
|
SnBu3 |
|
Y = Br |
|
|
|
|
|
SnBu3 |
|
|
|
SnBu3 |
|
|
|
B(OH)2 |
|
|
ZnCl |
Y = I |
||
B(OH)2 |
Y = Br |
||
B(OH)2 |
Y = Cl |
||
Pd(PPh3)4 |
73 |
[114] |
Pd(PPh3)2Cl2 |
74 |
[115] |
Pd(PPh3)2Cl2 |
0 |
[116] |
Pd(PPh3)4 |
50 |
[17] |
Pd(PPh3)4 |
79 |
[17] |
Pd(PPh3)4 |
0 |
[17] |
Pd(PPh3)4 |
16 |
[17] |
Pd(PPh3)4 |
56 |
[17] |
Pd(PPh3)4 |
0 |
[17] |
Cl2Pd(PPh3)2 |
15 |
[117] |
Cl2Pd(PPh3)2 |
21 |
[118] |
Pd(PPh3)4 |
67 |
[119] |
Ni(PPh3)4 |
93 |
[11] |
Pd2(dba)3, P(t-Bu)3 |
97 |
[87] |
Pd2(dba)3, P(t-Bu)3 |
93 |
[87] |
B(OH)2 |
Pd(PPh3)4, Ba(OH)2 |
94 |
[114] |
B(OH)2 |
Pd(PPh3)4, Ba(OH)2 |
56 |
[114] |
(Continued )

330 |
III |
Pd-CATALYZED CROSS-COUPLING |
|
|
|
|
||||||
TABLE 10. (Continued ) |
|
|
|
|
|
|
||||||
|
|
|
|
|
ArMX Ar'Y |
Cat. |
|
|
|
|||
|
|
|
|
|
9: Ar−Ar' |
|
|
|||||
Entry |
|
|
|
|
Substrates |
|
|
MX |
Catalyst |
Yield (%) |
Reference |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
11 |
|
|
|
|
|
|
|
Br |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MX |
Br |
|
|
MgBr |
NiBr2, (S)-PPFOMeb 89 |
[120] |
||
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Me |
|
|
|
|
|
|
|
|
|
MeO |
|
|
|
MeO |
|
|
|
|
|
|
|
12 |
BnO |
|
|
MX |
Br |
|
OMe |
B(OH)2 |
Pd(PPh3)4 |
90 |
[121] |
|
|
|
|
||||||||||
|
|
|
|
|
|
|
||||||
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
OMe |
MeO |
|
|
|
|
|
|
|
13 |
|
|
|
MX |
Br |
|
|
B(OH)2 |
Pd(PPh3)4 |
86 |
[122] |
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
Cr(CO)3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MOMO
aAryl electrophiles are arranged according to increasing order of priority determined by the Cahn–Ingold–Prelog rule.
b PPFOMe |
|
PPh2 |
|
|
Fe |
|
|
|
|
C |
OMe |
|
|
|
|
|
H |
|
Me |
|
|
|
|
TABLE 11. Pdor Ni-Catalyzed Aryl–Aryl Coupling Providing Biaryls Containing Four Ortho Substituents
|
|
|
ArMX Ar Y |
Cat. |
|
Ar – Ar |
|
|
||
|
|
|
9: |
|
|
|||||
|
|
|
|
|
|
|
|
|
||
Entry |
|
|
Substrates |
|
|
|
MX |
Catalyst |
Yield (%) |
Reference |
|
|
|
|
|
|
|
|
|
|
|
1 |
|
|
|
|
|
R = H |
MgBr |
Ni(PPh3)Cl2 |
55–79 |
[123] |
|
|
|
|
|
||||||
|
MX |
Br |
|
|
Me |
|||||
|
|
|
|
|
|
|
|
|||
|
|
|
R |
|
|
OMe |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ZnCl |
Ni(PPh3)Cl2 |
36 |
[124] |
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
||||
2 |
|
MX |
I |
|
|
|
ZnCl |
(CH3CN)2PdCl2 |
35 |
[124] |
|
|
|
|
|
B(OH)2 |
Pd(PPh3)4 |
39 |
[124] |
||
|
|
OEt |
MeO |
|
|
|
||||
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
||
|
|
MX |
Br |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
3 |
|
OMe |
N |
|
O |
|
B(OH)2 |
Pd(PPh3)4, Na2CO3 |
73 |
[125] |
|
|
|
||||||||
|
|
|
|
|
|
|
|
|||
N

