

IV.2.1.2 Double and Multiple Heck Reactions
STEFAN BRÄSE and ARMIN DE MEIJERE
A. INTRODUCTION
The multiple Pd-catalyzed couplings of oligohaloarenes and -alkenes (or perfluoroalkyland fluorosulfonates) with alkenes (Heck reactions) offer the possibility of constructing symmetrically oligosubstituted, highly unsaturated carbon frameworks in a single synthetic operation. The Heck reaction of diand oligoethenylarenes with diand oligohaloarenes can be applied to produce conjugated polyvinylenearylene-type polymers that may have important applications in the future. Comparable in scope and limitations to the related multiple Stille and Suzuki couplings, the ready accessibility of starting materials and the ease of removing by-products have made multiple Heck reactions particularly attractive for various synthetic applications.
In this section, a double (or multiple) Heck reaction is defined as a coupling of two (or more) alkene molecules with dior oligohaloarenes or -alkenes as well as a reaction of two (or more) dior oligohaloarenes or alkenes with one alkene molecule.
B. COUPLING OF OLIGOHALOARENES WITH ALKENES
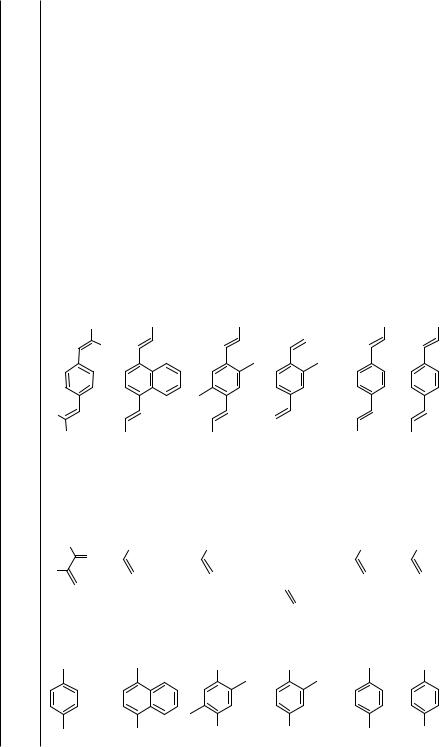
The multiple coupling of oligohaloarenes with alkenes was described early on by Heck and Nolley in one of their first papers.[1] Twofold coupling of 1,4-diiodobenzene with styrene furnished the 1,4-distyrylbenzene in 67% yield; shortly afterwards, the double Heck couplings under palladium catalysis of 4,7-diiodofluorene, p-diiodoterphenyl, and p- diiodobiphenyl with various substituted styrenes were disclosed by Japanese chemists in a patent on the synthesis of dye brighteners.[2] Since then, a large number of ortho-, meta-, and para-dihaloarenes and -heteroarenes have been subjected to double Heck reactions with various alkenes (Tables 1–3, Scheme 1).
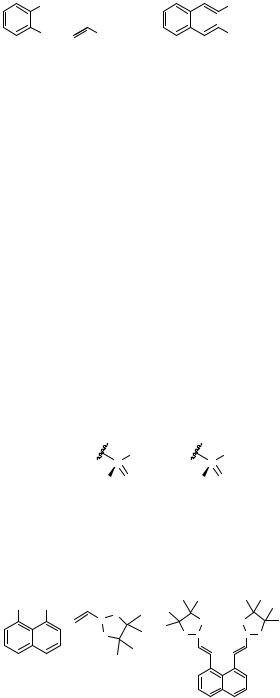
However, the substitution pattern on the arene is crucial for the success of the Heck reaction. When a second Heck coupling takes place in an ortho position of another alkenyl unit, cyclization of the intermediately formed -( -arylalkyl)palladium complex may occur, as formation of alkylideneindanes and alkylindenes, especially under classical Heck conditions with phosphines in the catalyst cocktail, was observed (Scheme 2, Table 1).
Handbook of Organopalladium Chemistry for Organic Synthesis, Edited by Ei-ichi Negishi ISBN 0-471-31506-0 © 2002 John Wiley & Sons, Inc.
1179
1180 |
IV |
Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
|
|
|||||
TABLE 1. Twofold Heck Reactions on 1,2-Dihaloarenes |
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
Arene |
|
Alkene |
|
Product |
|
|
|
|
|
|
X |
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
Yield |
Ref- |
|
X |
R |
|
|
R |
|
Conditions |
(%) |
erence |
|
|
|
|
|
|
|
|
|
|
X = I |
|
R = Ph |
|
R = Ph |
|
|
Pd(OAc)2, Bu3N, |
37 |
[1] |
|
|
|
|
|
|
|
100 °C, 72 h |
|
|
X = I |
|
R = CO2Me |
R = CO2Me |
|
Pd(OAc)2, PPh3, |
69 |
[7] |
||
|
|
|
|
|
|
|
Et3N, 100 °C, 48 h |
|
|
X = Br |
|
R = Ph |
|
R = Ph |
|
|
Pd(OAc)2, Bu4NBr, |
92 |
[8] |
|
|
|
|
|
|
|
K2CO3, DMF, |
|
|
|
|
|
|
|
|
|
100 °C, 5 d |
|
|
X = Br |
|
R = Ph |
|
R = Ph |
|
|
Pd(OAc)2, P(o-Tol)3, |
59 |
[9] |
|
|
|
|
|
|
|
Et3N, THF/MeCN, |
|
|
|
|
|
|
|
|
|
55 °C, 1 d, 10 kbar |
|
|
|
|
|
|
|
|
|
Pd(OAc)2, P(o-Tol)3, |
65 |
[10] |
|
|
|
|
|
|
|
Et3N, 150 °C |
|
|
|
|
|
|
|
|
|
(microwave), 22 min |
|
|
X = Br |
|
R = CO2Me |
R = CO2Me |
|
Pd(OAc)2, Bu4NCl, |
86 |
[11] |
||
|
|
|
|
|
|
|
LiCl, K2CO3, DMF, |
|
|
|
|
|
|
|
|
|
100 °C, 12 h |
|
|
X = Br |
|
R = Me (5 bar) |
R = Me |
|
|
Pd(OAc)2, Bu4NBr, |
79 |
[12] |
|
|
|
|
|
|
|
|
LiCl, KHCO3, |
|
|
|
|
|
|
|
|
|
DMF, 100 °C, 12 h |
|
|
X = Br |
|
R = H (13.8 bar) |
R = H |
|
|
Pd(OAc)2, P(o-Tol)3, |
78 |
[13] |
|
|
|
|
|
|
|
|
Et3N, MeCN, |
|
|
|
|
|
|
|
|
|
125 °C |
|
|
X = Br |
|
R = |
Ph |
R = |
Ph |
|
Pd(OAc)2, PPh3, |
65 |
[14] |
|
P |
|
P |
|
|
||||
|
|
Me |
O |
Me |
O |
|
Et3N, DMF, 135 °C, |
|
|
|
|
|
36 h |
|
|
||||
|
|
|
|
|
|
|
|
|
|
X = Br |
|
R = 4-Py |
|
R = 4-Py |
|
|
Pd(OAc)2, PPh3, |
80 |
[15] |
|
|
|
|
|
|
|
Et3N, 100 °C, 3 d |
|
|
X = Br |
|
R = Fc |
|
R = Fc |
|
|
Pd(OAc) , |
74a |
[16] |
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
(n-Bu)4NBr, K2CO3, |
|
|
|
|
|
|
|
|
|
DMF, 70 °C, 3 d |
|
|
I |
I |
O |
|
|
|
|
Pd(OAc)2, PPh3, |
77 |
[17] |
|
|
|
|
|
|
||||
|
|
B |
|
O |
|
O |
Et3N, MeCN, reflux |
|
|
|
|
O |
|
O BO |
|
B O |
|
|
|
|
|
|
|
16 h |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
IV.2.1.2 DOUBLE AND MULTIPLE HECK REACTIONS |
1181 |
|||
TABLE 1. (Continued ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Yield |
Ref- |
|
Arene |
Alkene |
|
Product |
Conditions |
(%) |
erence |
|
|
|
|
|
|
|
||
|
Ph |
|
Pd(OAc)2,Bu4NBr, |
35 |
[18] |
||
Br |
|
|
K2CO3, LiCl, DMF, |
|
|
|
|
|
|
100 °C, 3 d |
|
|
|
||
|
|
|
|
|
|
||
|
|
|
Ph |
|
|
|
|
|
Br |
|
Ph |
|
|
|
|
|
|
|
Pd(OAc)2, P(o-Tol)3, |
46 |
[19] |
||
|
|
|
DMF, 100 °C, 2 d |
|
|
|
|
|
|
OH |
O |
|
|
b |
|
|
I |
Pd(OAc)2, PPh3, |
56 |
[5] |
|||
|
|
||||||
|
|
|
|
||||
|
|
|
i-Pr2EtN, DMF, |
|
|
|
|
|
I |
|
100 |
°C, 3 d |
|
|
|
O
aFc = ferrocenyl.
bThe diketone was isolated.
R1
R1
X
X
|
X |
|
|
R2 |
R1 |
|
R2 |
||
|
,, Pd |
|
|
|
|||||
|
X |
,, |
|
|
|
R2 |
|||
|
|
|
|
||||||
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|||
|
X = Br, I; R2 = aryl, alkyl, etc. |
||||||||
|
|
|
|||||||
|
|
|
|
Scheme 1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R2 |
|
R2 |
R1 |
|
|
R2 |
R1 |
|||
,, |
,, |
|
|
|
|
|
|
|
|
|
Pd |
|
|
|
|
|
and/or |
|
R2 |
|
|
|
|
|
|
R2 |
|
|
|
Scheme 2
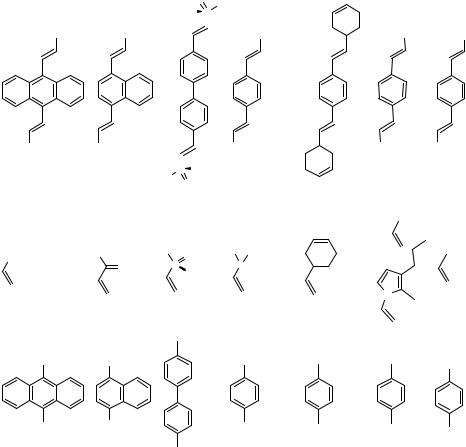
This reaction mode plays a dominant role in the sixfold Heck coupling of hexabromobenzene with styrenes yielding complex mixtures of various isomers of the sixfold coupling product. The analogous sixfold Suzuki and Stille coupling reactions with alkenylboronates and alkenylstannanes, respectively, gave the corresponding pure hexakisalkenylbenzene derivatives in high yields.[3] As shown by various experiments with o- and p-dihaloarenes, the second coupling step is generally accelerated by the first introduced alkenyl substituents in the ortho or para position, while a meta-alkenyl substituent does not significantly influence the rate of the second coupling.[4]
Yield (%) Reference
CO |
Product |
Me |
|
2 |
|
1,3-Dihaloarenes:E= |
Alkene (Conditions) |
Reactionson |
|
TwofoldHeck |
|
TABLE2. |
Arene |
[7]
a 46
E |
|
|
|
|
Br |
E |
|
|
|
N, |
|
|
3 |
|
E |
,Et |
|
3 |
|
|
PPh |
h) |
|
|
, |
100°C,19 |
|
2 |
|
|
(Pd(OAc) |
|
I |
|
Br |
I |
|
|
[20]
52
Bn 2 CO
C 2 BnO
NHBoc |
OBn |
I I
NHBoc
BocHN
NCl, 4 O Bu , 2 (Pd(OAc)
DMF, |
h) |
, |
|
3 |
|
NaHCO |
85°C,16 |
[21]
21
Ph
Ph
PhN,
3
Bu(Pd/C,
I I
115–120 °C, 3 h)
2 R
2 R
2 R
Br Br
[15] |
[14] |
77 |
92 |
|
R |
-4Py |
P(O)MePh |
|
|
1 |
|
|
|
|
|
|
|
= |
= |
|
|
|
|
2 |
2 |
|
|
|
|
=H,R |
=H,R |
|
|
|
|
1 |
1 |
|
|
|
|
R |
R |
|
|
|
|
|
|
N, |
|
|
|
|
|
3 |
|
|
|
, |
C,3d) |
,Et |
C,°36h) |
|
|
PPh, |
PPh, |
||
|
|
3 |
|
3 |
|
|
|
2 |
100° |
2 |
DMF,135 |
|
|
(Pd(OAc) |
Et |
(Pd(OAc) |
|
|
|
|
N, |
|
|
|
|
|
3 |
|
|
1 |
|
|
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
[22] |
|
b |
|
n.r. |
|
Et |
|
2 |
|
=CO |
|
2 |
|
=HOCHPh,R |
|
1 |
|
R |
|
, |
|
3 |
|
,P(o-Tol) |
MeCN) |
2 |
|
(Pd(OAc) |
Et |
|
N, |
|
3 |
[23]
94
Et |
|
2 |
|
OH,R=CO |
|
2 |
|
=CH |
|
1 |
|
R |
|
3 |
h) |
1 |
|
, |
|
o-Tol) 90°C, |
|
,P( |
MeCN, |
2 |
|
(Pd(OAc) |
Et |
|
N, |
|
3 |
[24]
56
=CN |
|
2 |
|
Et,R |
|
2 |
|
=CO |
|
1 |
|
R |
|
, |
7d) |
|
|
Tol)-o 120°C, |
|
3 |
|
,P( |
MeCN, |
2 |
|
(Pd(OAc) |
Et |
|
N, |
|
3 |
brominesubstituentdoesnottakepartinthecouplingundertheseconditions. |
=notreported. |
The |
n.r. |
a b |
|
1182
|
|
|
Reference |
|
|
Yield |
(%) |
2 |
|
|
Conditions |
Et |
|
|
|
Me,E =CO |
|
|
|
2 |
|
|
|
Dihaloarenes:E=CO |
|
|
Product |
para-Substituted |
|
|
|
1,4-andRelated |
|
|
Alkene |
TwofoldHeckReactionson |
|
|
Arene |
TABLE3. |
|
|
|
|
|
[1] |
|
|
[2] |
|
[25] |
|
|
[26] |
|
|
|
67 |
|
|
a |
11 |
|
|
76 |
|
|
|
|
|
|
n.r. |
|
|
|
|
|
||||
2 N, |
|
h |
|
|
2 |
N, |
°C, |
2 NCl, |
DMF,, |
|
h16 |
|
|
|
3 |
|
|||||||
|
|
|
|
|
|
3 |
|
|
|
|
|
, |
100°C,72 |
DMFPd, |
, |
Et, |
DMF,100 h17 |
, |
K |
|
800 |
||
Pd(OAc) Bu)-n( |
Pd(OAc) |
Tol)-oP( |
Pd(OAc) Bu)-n( |
|
|||||||
3 |
|
|
|
|
|
|
4 CO |
C, ° |
|||
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
-4-t-Bu |
|
|
|
|
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
Ph |
H |
|
|
Ph |
|
OR |
|
|||
|
C |
|
|
|
|
||||||
|
|
|
6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ph |
6 |
|
Ph |
RO |
||||||
|
|
|
4 |
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
||
|
|
|
-Bu-4-C |
|
|
|
|
|
||
|
|
|
t |
|
|
|
|
|
||
|
|
|
|
4--t-Bu |
Ph |
2 |
|
|||
|
Ph |
|
NMe |
|||||||
|
6 |
|
|
|
|
|
OR |
|||
|
|
|
4 |
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
C |
|
|
|
O |
||
|
|
|
|
|
|
|
|
^ = |
||
|
|
|
|
-4-I |
|
|
|
|
|
|
|
|
|
4 |
|
|
|
|
|
||
|
|
|
|
H |
|
|
|
|
|
|
|
I |
6 |
I |
|
I |
|||||
|
|
C |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4 |
|
|
I |
||
I |
H |
I |
|||||
|
|
6 |
|
|
|
|
|
|
|
4-I-C |
|
|
|
|
|
[7] |
|
|
32 |
|
|
, |
N, |
h |
2 |
3 |
100°C,48 |
Pd(OAc) |
PPh |
|
|
Et , |
|
|
3 |
|
E
E
E
I
I
[27] |
|
|
|
[28] |
(Continued) |
89 |
|
|
|
62 |
|
|
|
2:1, |
|
|
3 |
|
|
|
|
|
N, |
, |
, |
N/MeCN |
70°C,70h |
Pd(OAc),2 |
,Et |
2 |
3 |
3 |
|||
Pd(OAc) |
Tol)-P(o |
Et |
Tol)P(-o h60C,110° |
||
|
|
3 |
|
|
|
|
E |
|
OMe |
OMe |
|
|
|
|
|
OMe |
OMe |
OMe |
|
|
MeO |
MeO |
|
|
MeO |
|
E |
MeO |
MeO |
|
|
|
|
E |
OMe |
OMe |
OMe |
I |
OMe |
OMe |
Br |
|
MeO |
I |
MeO |
|
Br |
MeO |
1183
Yield (%) Reference
Conditions
Product
Alkene
3.(Continued) |
Arene |
TABLE |
|
55 [20]
, |
|
DMF, |
2 NCl, |
3 h |
|
Pd(OAc) Bu)-n( |
, |
|
NaHCO C,85°16 |
||
|
4 |
|
|
|
Bn |
|
|
2 |
|
|
CO |
|
|
BocHN |
BocHN |
2 |
|
|
C |
|
|
BnO |
|
NHBoc |
OBn |
O |
I
I
81 [21]
N, |
h |
3 |
3 |
Pd/C,(n-Bu) |
115–120°C, |
Ph
Ph
Ph
I
I
[18] |
[29] |
61 |
|
|
97 |
|
|
|
|
74 |
|
|
|
|
|
|
– |
|
|
, |
|
d |
|
N, |
h |
|
CO °C,5 |
|
Et, |
24 |
|
|
|
, |
|
3 |
|
2 NBr, |
3 |
Pd(OAc),2 |
|
–100120C,° |
|
2 |
3 |
||||
Pd(OAc) |
Bu)-n( |
LiCl,K DMF,110 |
P(-oTol) |
||
|
4 |
|
|
|
|
Ph
R
Ph
|
, |
|
2 |
Ph |
NO |
CN,H, C |
|
|
29 |
|
H |
|
10 |
|
2 |
|
R SO |
Br |
Br |
R
Br |
Br |
70 [30]
|
, |
|
|
3 |
5h |
2 CO |
||
, |
2 |
|
) |
|
|
(PPh |
N,K C,° |
|
3 |
|
|
2 |
3 O,100 |
|
PdCl |
Bu)n(- |
H |
|
|
2 |
Ph
Ph
Ph
I
I
[31]
85 |
|
|
|
2 |
, |
|
|
] |
3 |
h |
|
3 CO |
|
||
[P(o-Tol) |
N,K |
°C,6 |
|
|
2 |
|
|
2 |
3 O,100 |
||
PdCl |
Bu)n(- |
|
H |
|
|
|
2 |
Ph
Ph
Ph
Br
Br
1184
97 [30],[31]
, 3 -oTol)
P(, 2 PdCl
Ph
, |
|
3 |
3h |
CO |
|
K |
C,° |
2 |
|
N, |
100 |
3 |
|
Bu)-n( |
H |
|
O, |
|
2 |
Ph
Ph
Br
Br
72 [32]
, |
|
|
2 |
|
|
) |
DMF, |
24h |
(PPh |
||
3 |
|
|
2 |
N, |
130°C, |
PdCl |
Et |
|
|
3 |
|
E′
E′
O
OEt
OTf
TfO
[14] |
[33] |
85 |
39 |
, |
|
|
|
|
3 |
|
|
|
|
,PPh |
DMF, |
h |
||
2 |
||||
Pd(OAc) |
Et |
135°C,36 |
||
|
N, |
|
|
|
|
3 |
|
|
|
|
O |
|||
|
Me |
|
P |
|
|
|
|||
|
|
|
||
|
|
|
|
|
|
|
|
Me |
|
P |
||||
|
||||
Ph |
O |
|||
Ph |
O |
|
P |
|
Me |
I
I
|
, |
|
|
|
3 |
|
|
|
,PPh |
MeCN, |
h |
|
2 |
||
|
Pd(OAc) |
Et |
125°C,24 |
|
|
N, |
|
|
|
3 |
|
Ph |
|
2 |
|
P(O)Ph |
|
||
(O)P 2 Ph
Ph |
|
Ph |
|
|
P |
|
O |
|
|
||
|
|
||
Br
Br
21 [34]
2 |
NCl, |
, |
BnEt |
Pd(OAc) |
|
|
3 |
I
I
82 [35]
|
N, |
|
KOAc,25°C, d10 |
3 |
h6 |
PdCl 80DMF,°C, |
||
|
Bu)-n( |
|
|
, |
|
|
2 |
|
Ar
Ar
^ = Ar
N
I
I
[36] |
|
|
(Continued) |
b |
|
|
|
42 |
|
|
|
, |
MeCN, |
|
|
2 |
|
h1 |
|
(PPh |
|
||
) |
|
|
|
3 |
|
|
|
2 |
N, |
|
80°C, |
PdCl |
Et |
|
|
|
3 |
|
|
|
|
Et |
|
|
|
|
2 |
|
|
CO |
|
C 2 EtO
Et 2 CO
Br
I
1185
Yield (%) Reference
Conditions
Product
Alkene
3.(Continued) |
Arene |
TABLE |
|
[37] |
|
|
c |
|
|
90 |
|
|
|
N, |
h |
2 |
3 |
5 |
3 |
C,° |
|
, |
Et |
|
Pd(OAc) |
, |
DMF,80 |
P(o-Tol) |
Ph
OHept
RO
Ph
Me
2
SO
59 [20]
, 2 Pd(OAc)
NHBoc
NCl, |
DMF,, |
|
3 h |
||
Bu)-n( |
NaHCO |
C,85°16 |
4 |
|
|
|
|
Bn |
|
|
2 |
|
|
CO |
BocHN
C 2 BnO
Ph |
NHBoc |
OBn |
O |
N |
|
|
|
Et |
|
|
|
= |
|
I |
|
R |
|
|
|
I |
OHept |
|
|
RO |
|
|
|
I |
|
I |
|
24 [38]
2 NBr, |
|
, |
Bu)-n( |
Pd(OAc) |
|
|
4 |
Fc
Fc
Br
DMF, |
d |
3 |
3 |
, |
|
CO °C, |
|
2 |
70 |
K |
|
Fc
Br
[39] |
|
|
|
|
|
|
|
|
74 |
|
|
|
|
|
|
|
|
|
|
N, °C |
|
|
|
|
||
|
|
3 |
120 |
|
|
|
|
|
, |
|
Et, |
– |
|
|
|
|
|
2 |
3 |
DMF,100 |
|
|
|
|
||
Pd(OAc) |
P(o-Tol) |
|
|
|
|
|||
|
|
|
|
|
|
|
in12%yield. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BrBr |
|
reported.not=n.r. |
productmonosubstitutionThe(iodinereplaced)wasisolatedin22%yield. products.couplingofyieldTotal Themono-α-substitutedstyrenewasisolated |
ferrocenyl.=Fc |
||||
|
|
bar)(30 |
|
|
|
|
|
|
|
|
|
|
|
a |
b |
c |
d |
|
|
|
|
|
||||
|
|
|
|
|
||||
|
|
|
|
|
||||
|
|
|
|
|
||||
|
|
|
|
|
||||
|
|
|
|
|
||||
1186
IV.2.1.2 DOUBLE AND MULTIPLE HECK REACTIONS |
1187 |
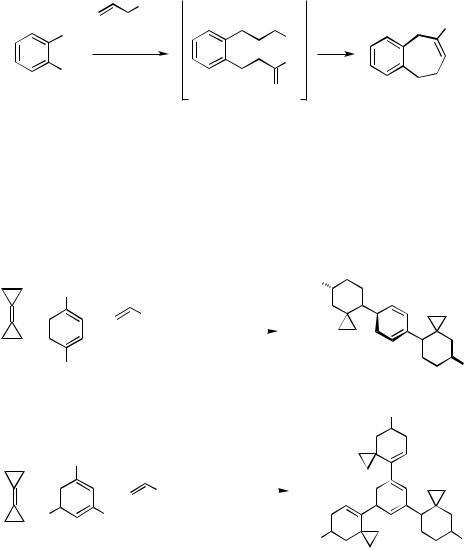
The primary coupling product of 1,2-diiodobenzene with allyl alcohol, a dicarbaldehyde, underwent an intramolecular aldol condensation under the reaction conditions (Scheme 3).[5]
OH |
|
O |
|
|
||
|
|
|||||
I Pd(OAc)2, Et3N |
|
|
|
|
|
CHO |
|
|
|
|
|
|
|
80 °C, 44 h |
|
|
|
H |
||
H 81%
I
O
Scheme 3
Double and even triple Heck–Diels–Alder cascade reactions involving bicyclopropylidene and 1,4-diiodo- or 1,3,5-triiodobenzene, respectively, have been accomplished. In these sequences, the carbopalladation across the highly strained alkene is followed by a cyclopropylmethyl to homoallyl rearrangement with concomitant -hydride elimination to yield an allylidenecyclopropane, which subsequently undergoes a smooth [4 2] cycloaddition to furnish the spiro[5.2]octene moiety (Scheme 4).[6]
|
|
I |
|
|
|
|
|
MeO2C |
|
|
||||
|
|
|
|
Pd(OAc)2, PPh3, |
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
Bu4NCl, K2CO3, |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|||||||
+ |
|
|
+ |
CO2Me MeCN, 80 °C, 2 d |
|
|
||||||||
|
|
|
|
|||||||||||
|
|
|
|
|||||||||||
|
|
I |
|
|
64% |
|
|
|
|
|
|
|
CO2Me |
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
CO2Me |
|
|
|
|
X |
|
|
Pd(OAc)2, PPh3, |
|
|
||||||
|
|
|
|
|
Bu4NCl, K2CO3, |
|
|
|||||||
|
|
|
|
|
|
|
|
|||||||
+ |
|
|
+ |
|
|
MeCN, 80 °C, 2 d |
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
CO2Me |
|
|
|
|
||||||
|
|
|
|
|
|
X = Br: 34% |
|
|
|
|
||||
X |
X |
|
|
|
|
|
||||||||
|
|
X = I: 72% |
|
|
||||||||||
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
MeO2C |
|
CO2Me |
||||
|
|
|
|
|
|
|
|
|
||||||
Scheme 4
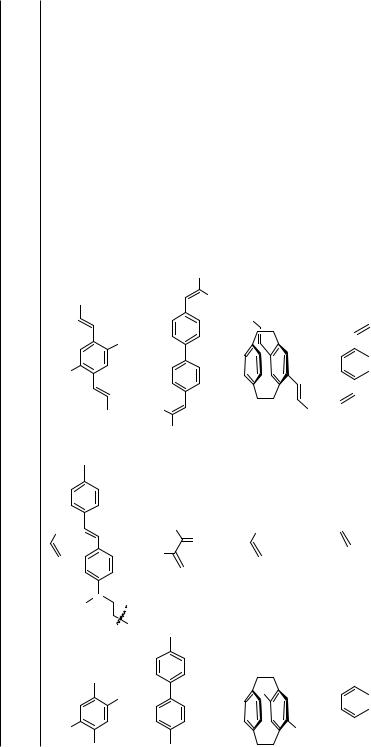
In later studies by Heck and co-workers,[7] extension to threefold and higher multifold couplings with the corresponding oligoiodoarenes failed, and only major fractions of reduced starting materials were observed. This drawback was completely overcome by applying the Jeffery protocol,[40] that is, with a base like potassium carbonate in the presence of a tetrabutylammonium halide.[41],[42] Under these conditions, three alkene units and more can be attached to an arene ring in excellent yields (Tables 4 and 5).
1188 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
TABLE 4. Threefold Heck Reaction on 1,2,3-, 1,2,4-, and 1,3,5-Trihaloarenes: E = CO2Me, Fc = Ferrocenyl
|
Arene |
|
|
|
|
|
|
|
|
|
Yield |
Ref- |
||||||
|
Alkene |
Product |
|
|
Conditions |
(%) |
erence |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
I |
E |
|
|
Pd(OAc)2, PPh3, |
52 |
[7] |
||||||||
|
|
|
|
|
|
|
I |
|
|
|
|
|
|
|
E |
Et3N, 100 °C, 12 h |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
I |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
CO2Me |
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
E |
|
|
|
|
|
|||||
Br |
|
|
|
|
|
|
Br |
R |
|
R |
Pd(OAc)2, |
46a |
[16] |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(n-Bu)4NBr, K2CO3, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DMF, 2 h, 60 °C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Br |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
|
|
|
|
|
|
Fc |
|
|
|
|
|
|
|
R = Fc |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4-Py |
|
|
|
R = 4-Py |
||||||||
|
|
2-Py |
|
|
|
R = 2-Py |
||||||||
|
|
|
|
|
|
|
|
|
|
R = H |
||||
(8 bar) |
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
Ph |
|
|
|
|
|
|
|
R = Ph |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C H |
-4-OMe |
|
R = C6H4-4-OMe |
||||||||||
6 |
4 |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
Br |
|
|
|
|
|
|
|
|
|
Ph |
||
|
|
|
|
|
|
Ph |
|
|
|
|
|
|
|
|
Br |
|
|
Br |
|
|
|
|
|
|
|
Ph |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
X X = H, NO2 |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
Ph |
|
|
|
|
|
X |
|||
|
|
|
|
|
|
|
|
|
|
R |
||||
|
|
Br |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Br |
|
|
Br |
R |
|
|
|
|
|
|
|
R |
||
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Pd(OAc)2, PPh3, |
70 |
[43] |
Et3N, 100 °C, 3 d |
(60) |
([15]) |
Pd(OAc)2, K2CO3, |
92 |
[44] |
(n-Bu)4NBr, DMF, |
|
|
100 °C, 5 d |
|
|
Pd(OAc)2, |
71 |
[45] |
P(o-Tol)3, Et3N, |
|
|
DMF, 125 °C |
|
|
Pd(OAc)2, |
82 |
[4] |
(n-Bu)4NCl, K2CO3, |
|
|
LiCl, DMF, 110 °C, |
|
|
30 h |
|
|
Pd(OAc)2, PPh3, |
>40 |
[43] |
Et3N, 100 °C, 3 d |
|
|
Pd(OAc)2, |
56 |
[8] |
(n-Bu)4NBr, K2CO3, |
X = H |
|
DMF, 100 °C, 7 d |
|
|
Pd(OAc)2, |
66 |
[4] |
(n-Bu)4NCl, K2CO3, |
X = NO2 |
|
LiCl, DMF, 100 °C, |
|
|
4 d |
|
|
Pd(OAc)2, |
58 |
[11] |
(n-Bu)4NCl, K2CO3, |
|
|
LiCl, DMF, 3 d, |
|
|
90 °C |
|
|
CO2t-Bu |
R = CO2t-Bu |
