

RI + Et2Zn |
cat. Pd |
RZnI |
|
IV.11 Palladium-Catalyzed
Carbozincation
PAUL KNOCHEL
A. INTRODUCTION
The carbopalladation is a central reaction in organopalladium chemistry and is extensively presented in Part IV. In most reactions, a discrete organopalladium intermediate adds to a double or triple bond. In this section, the reaction of an alkyl iodide with diethylzinc in the presence of a palladium(0) catalyst is presented. Such reaction conditions generate an alkyl radical that readily adds intramolecularly to a double bond, leading to an organozinc derivative (Scheme 1). The combination of a radical cyclization with the formation of an organometallic product allows new synthetic applications that will be discussed. Closely related Ni-catalyzed cyclizations will also be briefly presented.
B. MECHANISTIC STUDIES
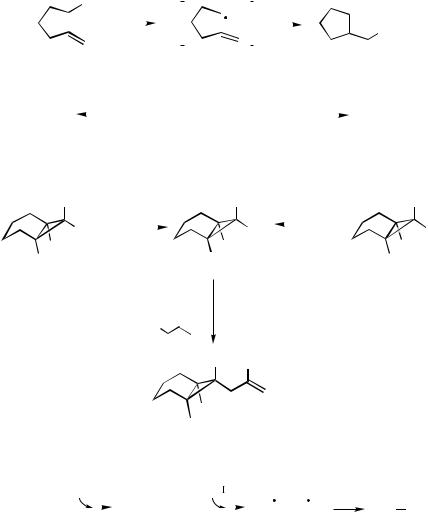
The iodine–zinc exchange[1],[2] is a convenient method for preparing diorganozincs. It is catalyzed by copper(I) salts[2] and proceeds at temperatures between 50 and 60 °C. Other metallic salts catalyze this exchange reaction and especially palladium(II) salts give excellent results, allowing the conversion of octyl iodide to octylzinc iodide (and not dioctylzinc as shown by gravimetric analysis)[3] (Scheme 2). Functionalized primary alkyl iodides such as EtO2C(CH2)3I or NC(CH2)3I react within 1 0 – 30 min at 25 °C ( 90% yield) without the formation of any -hydride elimination product. In order to clarify the mechanism of the reaction, other precursors were used. It was found that alkyl tosylates or mesylates do not react. The reaction is inhibited by small amounts of nitrobenzene and does not proceed with Me2Zn. The exchange reaction is also very slow in ether. The treatment of either exo- or endo-7-iodobicyclo[2.1.0]heptane[4] with Et2Zn (ca. 2 equiv) in the presence of PdCl2(dppf) in THF furnishes the exo-substituted bicyclic organozinc reagent, which was allylated in a stereoconvergent manner, suggesting a radical to be a reaction intermediate (Scheme 3).
The insertion of Pd(0) into an alkyl iodide may therefore proceed via a radical pathway.[5],[6] The fast oxidative insertion may also indicate that a palladate species[7] such as 1 may be responsible for an electron transfer reaction[8] (Scheme 4). By using 5-hexenyl
Handbook of Organopalladium Chemistry for Organic Synthesis, Edited by Ei-ichi Negishi ISBN 0-471-31506-0 © 2002 John Wiley & Sons, Inc.
1651
1652 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
|
||||||||||
|
I |
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|||||
|
|
|
Et2Zn |
|
|
|
|
|
|
|
|
|
|
|
|
PdCl2L2 |
|
|
|
|
|
|
ZnI |
||
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
Scheme 1 |
|
|||||
|
Et2Zn, 50 °C, 12 h |
|
Et2Zn, THF, 25 °C, 2 h |
|
||||||||
|
Oct2Zn |
|
|
Oct-I |
|
|
Oct-ZnI |
|||||
|
CuI (0.3 mol %) |
PdCl2(dppf) (1.5 mol %) |
||||||||||
|
|
|
|
|||||||||
−H2C=CH2 −H3C CH3
Scheme 2
I |
|
H |
||||
|
Et2Zn, Pd(0) cat. |
|
Et2Zn, Pd(0) cat. |
|||
H |
|
|
|
ZnI |
|
|
|
|
|||||
H |
H |
H |
||||
H |
exo/endo = 96:4 |
|||||
|
|
|
|
|||
endo
1.CuCN 2 LiCl
2.COOEt Br 
|
|
|
|
|
|
|
|
|
|
H |
COOEt |
|||
|
|
|
|
|
|
|
|
|
H |
60% |
||||
|
|
|
|
|
|
|
|
|
H |
|||||
|
|
|
|
|
|
|
|
|
Scheme 3 |
|
||||
|
Et2Zn |
|
|
|
|
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Pd(0) |
|
EtPd ZnEt |
|
|
|
|
|
EtPd + R |
||||||
|
|
−EtZnI |
||||||||||||
|
|
1 |
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
Scheme 4 |
|
||||
H
I
H
H exo
EtPd R
iodide as a substrate, a radical cyclization occurs, affording cyclopentylmethylzinc iodide (Scheme 1). This synthetic procedure allows one to perform radical ring closures[9] but affords as a product an organometallic species (organozinc halide) that can be used to form further carbon–carbon bonds. The stereoselectivity observed in the ring closure follows Beckwith rules[10] and is also stereoconvergent. Thus, the cyclization of the iodides 2a and 2b provides only the trans-product via a chair transition state such as 4
(Scheme 5).
A complete trans-stereoselectivity is observed between C(1) and C(2) of products 3a-b. The cyclization leads as expected[10] to lower stereoselectivity for the ring closure, affording the allylated product 5 as a cis/trans mixture of 78:22. This selectivity can dramatically be improved by using the 3-substituted secondary alkyl iodide 6. Although
H
I Et2Zn (2 equiv)
RO
PdCl2(dppf) 1.5 mol % 25 °C, 20 h
2a: R = Bn
2b: R = Bz
IV.11 |
PALLADIUM-CATALYZED CARBOZINCATION |
1653 |
||||||
|
|
|
|
|
|
|
EtO2C |
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
99:1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
1. CuCN • 2 LiCl |
RO |
|
||||
RO |
H |
|
||||||
|
|
|
|
|||||
|
COOEt |
|
|
67% |
||||
|
|
|
|
|
|
|||
|
H H |
2. |
|
|
|
|||
|
|
Br |
3a: R = Bn 73% |
|
||||
|
4 |
|
|
|
|
3b: R = Bz 47% |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
Scheme 5 |
|
|
|
|
|
|
|
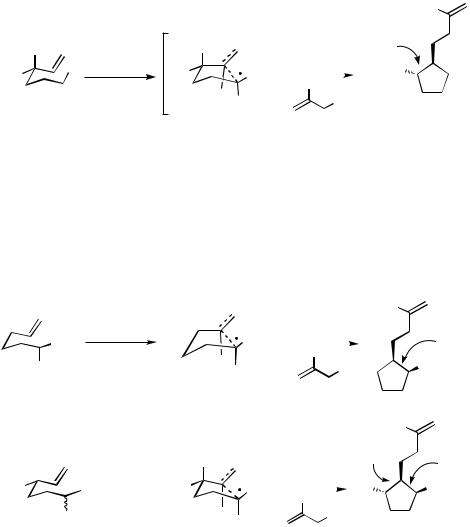
6 is used as a 1:1 mixture of diastereoisomers, only one stereoisomer 7 is obtained. Its formation is easily rationalized by assuming a chair transition state such as 8 (Scheme 6).[4],[11] The stereoconvergence of the reaction supports the radical mechanism of the ring closure.
Et2Zn (2 equiv)
Me
PdCl2(dppf)
I |
1.5 mol % |
||
25 |
°C, 2 h |
||
|
|||
Et2Zn (2 equiv)
BnO I 
PdCl2(dppf)
Me |
1.5 mol % |
||
25 |
°C, 5 h |
||
|
|||
6
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EtO2C |
|
|||
|
|
|
|
|
|
|
|
|
|
1. CuCN • 2 LiCl |
|
|
|
|||||
|
|
|
|
Me |
|
|
|
|
|
78:22 |
||||||||
|
|
|
|
H |
|
|
|
|
COOEt |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
2. |
Br |
|
Me |
|
|||||||
|
|
|
|
H |
|
|
|
|
|
5 |
80% |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EtO2C |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
99:1 |
|
|
|
||||
|
|
|
|
H |
|
|
|
95:5 |
||||||||||
|
|
|
|
|
1. CuCN • 2 LiCl |
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
Me |
|
||||||||
BnO |
Me |
|
|
|
|
|
|
BnO |
|
|
||||||||
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
2. |
|
COOEt |
|
|
|
|
||||
|
|
|
|
H H |
|
|
Br |
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
7 67% |
|
|
||||||||
|
|
|
|
8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 6 |
|
|
|
|
|
|
|
|
|
|
|
|
||
C. SCOPE AND LIMITATION OF THE CYCLIZATION
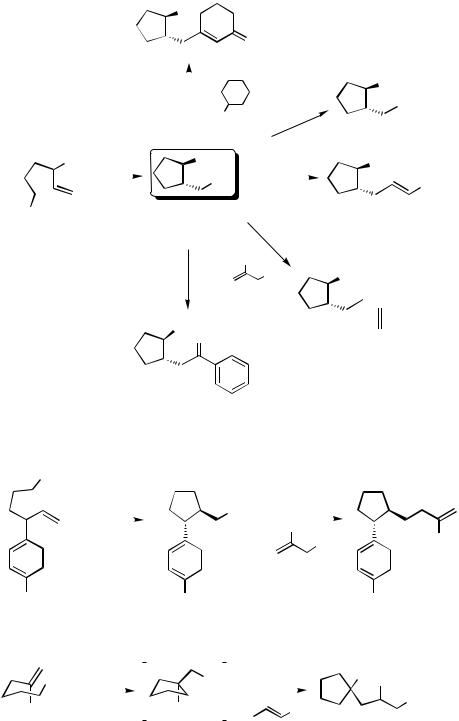
The cyclization of 4-substituted 5-hexenyl iodides proceeds well, leading to cyclopentylzinc iodides that can be trapped with various electrophiles such as 3-iodo-2-cyclohexenone, iodine, acid chlorides, allylic halides, and ethyl propiolate (carbocupration) (Scheme 7).[4]
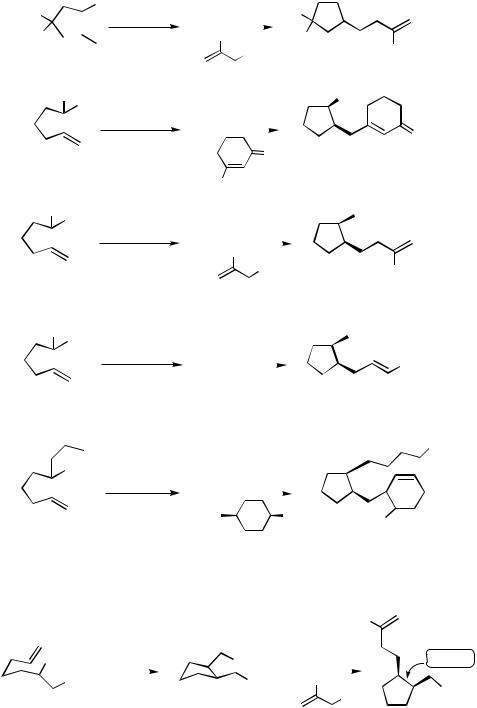
Various substitution patterns allow a successful cyclization. Also, a range of functional groups like esters or nitriles are tolerated in the ring closure (Scheme 8).
The presence of an oxygen functionality (O-centered leaving group) is also compatible with the reaction conditions and the 2-pivaloyloxyalkyl iodide 9 leads after allylation to the expected cyclopentane derivative 10 (Scheme 9).[4]
1654 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
|||||||||||||||
|
|
|
|
Ph |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
||||
|
|
|
|
|
1. CuCN • 2 LiCl |
|
|
|
Ph |
|||||||
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
2. |
|
|
|
|
|
O |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
I2 |
I |
||||
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
I |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
90% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ph |
Et2Zn, THF |
|
Ph |
|
|
|
|
|
|
|
|
|
|
|
|
|
25 °C, 2h |
|
|
|
|
1. CuCN • 2 LiCl |
Ph |
|||||||||
|
|
|
|
|
||||||||||||
|
|
PdCl2(dppf) |
|
ZnI |
|
|
|
|
|
CO2Et |
|
CO2Et |
||||
|
|
|
|
2. |
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
||||||||||
|
|
1.5 mol % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
I |
|
|
>98% trans |
|
|
|
|
|
|
|
|
|
|
64% >95% E |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
1. CuCN • 2 LiCl |
|||
|
|
1. CuCN • 2 LiCl |
2. CO2Et |
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
Br |
Ph |
|||||||
|
|
2. PhCOCl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 CO2Et
CO2Et
Ph
O
73%
76%
Scheme 7
I
|
|
|
Et2Zn (2 equiv) |
|
|
|
|
|
|
|
|
1. CuCN • 2 LiCl |
|
|
|||||||
|
|
|
|
|
|
|
ZnI |
|
|
|
|||||||||||
|
|
|
PdCl2(dppf ) |
|
|
|
|
|
|
|
|
|
CO2Et |
|
|
|
|
||||
|
|
|
1.5 mol % |
|
|
|
|
|
|
2. |
|
Br |
|
CO2Et |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
25 °C, 2 h |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
FG |
|
|
|
|
|
|
|
FG |
|
|
|
|
|
|
|
|
|
|
FG |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FG = CN 83% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FG = OCOt-Bu 62% |
|
|
I |
|
|
|
|
|
ZnI |
|
1. CuCN • 2 LiCl |
|
|
Bu Ph |
|||||||||
|
Et2Zn (2 equiv) |
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bu |
PdCl2(dppf ) |
|
|
|
Bu |
|
|
2. |
|
|
NO2 |
|
|
|
NO2 |
||||||
1.5 mol % |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
25 °C, 2 h |
|
|
|
|
|
|
|
Ph |
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
||||||||||||
81%
Scheme 8
IV.11 PALLADIUM-CATALYZED CARBOZINCATION |
1655 |
I
E |
Et2Zn (2 equiv) |
|
E PdCl2(dppf )
PdCl2(dppf )
1.5mol % 25 °C, 2 h
E = CO2Et
R
I
Et2Zn (2 equiv)
PdCl2(dppf ) 1.5 mol % 25 °C, 2 h
R = Me, Et, c-Hex
R
I
Et2Zn (2 equiv)
PdCl2(dppf ) 1.5 mol % 25 °C, 2 h
R = (CH2)4OAc, Et, (CH2)3CN
R
I
Et2Zn (2 equiv)
PdCl2(dppf ) 1.5 mol % 25 °C, 2 h
R = (CH2)4OAc, (CH2)3CN
 OAc
OAc
I
Et2Zn (2 equiv)
PdCl2(dppf ) 1.5 mol % 25 °C, 2 h
|
1. CuCN • 2 LiCl |
E |
|
|||||||
|
|
|
||||||||
|
2. |
|
CO2Et |
|
E |
73% |
||||
|
|
|
|
Br |
|
CO2Et |
||||
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
E = CO2Et |
|
|
|
|
|
|
|
|
|
|
R |
|
|
1. CuCN • 2 LiCl |
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
O |
|
2. |
|
|
|
|
O |
|
|||
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
62−81% |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
I |
cis/trans ca. 70:30 |
||||||
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
R |
|
|
1. CuCN • 2 LiCl |
|
|
|||||||
|
2. |
|
COOEt |
|
|
|||||
|
|
|
|
|
Br |
|
CO2Et |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
75−87% |
|
|
|
|
|
|
|
|
|
|
cis/trans |
ca. 78:22 |
|
|
|
|
|
|
|
|
|
R |
|
|
1. CuCN • 2 LiCl |
|
|
CO2Et |
||||||
|
2. |
|
|
|
CO2Et |
|
||||
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|||||
|
|
|
|
|
||||||
51−71% cis/trans ca. 78:22
OAc
1. CuCN • 2 LiCl |
|
|
||
2. |
|
|
|
|
|
|
|
|
|
Cl |
OAc |
AcO |
||
|
|
|
|
|
52% cis/trans 77:23
Scheme 8 (Continued )
|
|
|
|
EtO2C |
|
|
I |
Et2Zn |
|
ZnI |
1. CuCN • 2 LiCl |
80:20 |
|
OPiv |
|
|
|
CO2Et |
|
|
PdCl2(dppf ) |
OPiv |
|
||||
|
1.5 mol % |
|
2. |
|
OPiv |
|
|
|
Br |
||||
|
25 °C, 2 h |
|
|
|||
|
|
|
|
|
||
9
10 87%
Scheme 9
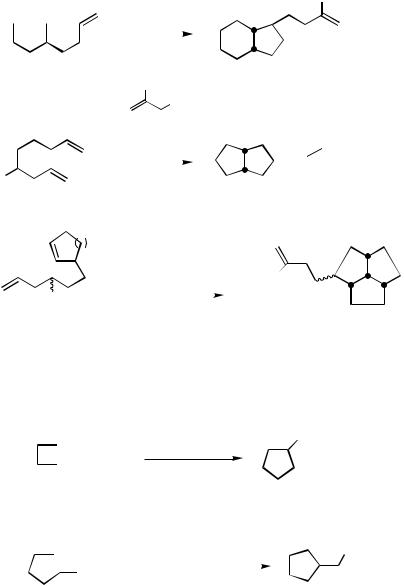
1656 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
Various domino cyclizations using substrates like 1 1–13 have been used successfully (Scheme 10).[4],[11]
The intramolecular addition to unsaturated esters was also possible. Best results are obtained with a t-amyl ester, which leads to a product that is less prone to undergo Claisen condensation. The same reaction is observed with the corresponding acetylenic ester (Scheme 11).[4]
 I
I
1. Et2Zn |
|
CO2Et |
PdCl2(dppf ) cat. |
|
|
25 °C, 2 h |
|
|
2. CuCN • 2 LiCl |
|
|
COOEt |
90% endo/exo 80:20 |
|
Br |
|
|
|
1. Et2Zn |
|||||
|
PdCl2(dppf ) cat. |
|||||
I |
|
25 °C, 2 h |
|
|||
2. CuCN • 2 LiCl |
||||||
11 |
|
|
|
CO2Et |
||
|
|
|||||
|
|
|
||||
|
|
|||||
|
|
|
|
|
|
|
n |
1. Et2Zn (2 equiv) |
|||||
|
Ni(acac)2 (2.5 mol %) |
|||||
|
|
0 °C, 3h |
||||
I |
2. CuCN • 2 LiCl |
|
||||
|
|
|
CO2Et |
|||
|
|
|
|
|||
12n = 1
13n = 2
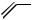 CO2R
CO2R
 I
I
 Br
Br
Scheme 10
Et2Zn, THF PdCl2(MeCN)2 (1.5 mol %)
−78 °C to 25 °C, 4 h

 CO2Et
CO2Et
70% endo/exo 80:20
EtO2C
n = 1: 85 % endo/exo 1:2 n = 2: 63 % endo/exo 1:2
CH2 CO2R
R = Et |
R = Et 57% |
|
|||||
R = t-Am |
R = t-Am 74% |
|
|||||
|
|
|
CO2Me |
Et2Zn ( 2 equiv), THF |
|
CO2Me |
|
|
|
|
|||||
|
|
|
|
||||
|
|
|
|
||||
|
|
I |
|
|
|
||
|
|
PdCl2(MeCN)2 ( 1.5 mol % ) |
|
||||
|
|
|
|
25 °C, 4 h |
|
||
|
|
|
|
73% |
|
|
|
|
|
|
|
Scheme 11 |
|
||
IV.11 PALLADIUM-CATALYZED CARBOZINCATION |
1657 |
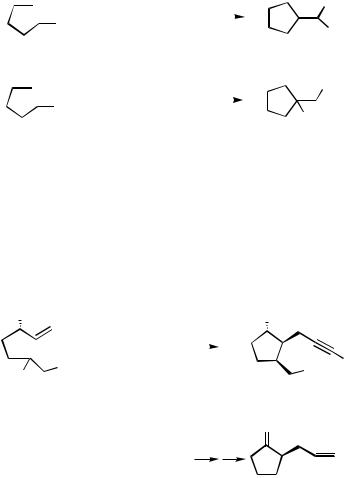
The reactivity observed with acetylenic ketones is more complex and the two iodoalkynyl ketones 14 and 15 behave in a different way (Scheme 12). Thus, the phenyl ketone 14 undergoes carbopalladation of the triple bond followed by a reductive elimination, furnishing the exo-alkylidenecyclopentane derivative 16. On the other hand, the methyl ketone 15 undergoes, after carbopalladation, a subsequent Michael addition, leading to the ketone 17 in 52% yield.
|
|
|
|
|
|
COPh |
Et2Zn, THF |
|
|
COPh |
||
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
I |
|
|
|
|
|
|
||
|
|
|
|
PdCl2(MeCN)2 (1.5 mol %) |
|
|
Et |
|||||
|
|
|
|
|
|
|
25 °C, 4 h |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
14 |
|
|
|
|
|
16 |
60% |
|||||
|
|
|
|
|
COMe |
Et2Zn, THF |
|
|
COMe |
|||
|
|
|
|
|
||||||||
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|||||
|
|
|
|
I |
|
|
|
|
|
|||
|
|
|
|
PdCl2(MeCN)2 (1.5 mol %) |
|
Et |
||||||
|
|
|
|
|
|
|
25 °C, 4 h |
|
||||
15 |
|
|
|
|
|
|
17 52% |
|||||
Scheme 12
The scope of the reaction can be extended to unsaturated alkyl bromides as substrates by using Ni(acac)2 as a catalyst[12],[13] instead of Pd(II) complexes. The use of Ni(acac)2 as a catalyst even with several polyfunctional alkyl iodides gives better results. Thus, in the key step for the synthesis of methyl epijasmonate 18, the alkyl iodide 19 undergoes a smooth cyclization using Ni(acac)2 and Et2Zn (Scheme 13).[12]
OBn |
1. Et2Zn, Ni(acac)2 cat |
OBn |
|||||
|
|
||||||
|
THF, 25 °C |
|
|||||
CO2Me |
2. CuCN • 2 LiCl |
Et |
|||||
3. Br |
|
|
|
|
Et |
||
|
|
|
|
||||
|
|
|
|
CO2Me |
|||
|
|
||||||
I |
−55 °C, 48 h |
|
|||||
19 |
|
|
|
|
|
|
86% |
|
|
|
|
|
|
95:5 |
|
|
|
|
|
|
|
|
|
O
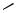 Et
Et
 CO2Me
CO2Me
18 epijasmonate
Scheme 13
Polyfunctional alkyl bromides have been cyclized with Ni(acac)2/Et2Zn for the construction of various heterocycles[13] as well as for the antitumor antibiotic ( )-methylenolactocin 20. In this case, the alkyl bromide 21 is cyclized and selectively oxidized to the aldehyde 22, which is converted in a standard way to the natural product
20 (Scheme 14).
1658 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
|
|
|||||||||||||||||||||||
Me3Si |
|
Br |
|
1. Et2Zn, LiI |
|
|
|
|
ZnX |
|
|
|
|
|
|
|
|
|
OHC |
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
Ni(acac)2 |
|
|
O |
|
SiMe3 |
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
THF, 40 °C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
C |
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Pent |
O |
OBu |
|
2. O2, TMSCl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pent |
O |
OBu |
||
|
THF, −5 °C |
|
|
|
|
|
|
|
|
|
OBu |
|
|
|
|
|
|
|||||||||
|
21 |
|
|
|
|
|
|
Pent |
|
O |
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
22 |
55% |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
HO2C |
|
|
|
|
|
|
|
|
|
|
|
HO2C |
|
||||||
|
|
|
|
Jones reagent |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
acetone, |
Pent |
|
O |
O |
|
|
|
|
|
|
|
|
Pent |
O |
O |
||||||
|
|
|
|
0 °C, 15 min |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
90 % |
|
|
|
|
|
|
20: (−)-methylenolactocin |
||||||
|
|
|
|
|
|
|
|
Scheme 14 |
|
|
|
|
|
|
|
|
|
|
|
|
||||||
D.SUMMARY
1.The treatment of an alkyl iodide with Et2Zn in the presence of catalytic amounts of palladium(II) salts leads to the corresponding organozinc iodide.
2.The reaction proceeds via a radical intermediate and can be used to perform radical cyclizations affording five-membered rings. However, the products of these reactions are organozinc reagents, which can be reacted with a wide range of electrophiles.
3.The cyclizations are stereoselective following the Beckwith rules and allow the elaboration of highly substituted cyclopentane derivatives.
4.Domino cyclizations can be performed.
5.Unsaturated alkyl bromides can be used as substrates if the Pd(II) catalyst is replaced by Ni(acac)2.
REFERENCES
[1]M. J. Rozema, S. AchyuthaRao, and P. Knochel, J. Org. Chem., 1992, 57, 1956.
[2]M. J. Rozema, C. Eisenberg, H. Lütjens, R. Ostwald, K. Belyk, and P. Knochel, Tetrahedron Lett., 1993, 34, 3115.
[3]H. Stadtmüller, R. Lentz, W. Dörner, T. Stüdemann, C. E. Tucker, and P. Knochel, J. Am. Chem. Soc., 1993, 115, 7027.
[4]H. Stadtmüller, A. Vaupel, C. E. Tucker, T. Stüdemann, and P. Knochel, Chem. Eur. J., 1996, 2, 1204.
[5]A. V. Kramer, J. A. Labinger, J. S. Bradley, and J. A. Osborn, J. Am. Chem. Soc., 1974, 96, 7145.
[6]A. V. Kramer and J. A. Osborn, J. Am. Chem. Soc., 1974, 96, 7832.
[7]C. Amatore, E. Carré, A. Jutand, H. Tanaka, Q. Ren, and S. Torii, Chem. Eur. J., 1996, 2, 957.
[8]M. Chanon, Bull. Soc. Chim. Fr., 1982, 2, 197.
[9]D. P. Curran, in Comprehensive Organic Chemistry, Vol. 4, B. M. Trost and I. Fleming, Eds., Pergamon Press, New York, 1991, 779.
IV.11 PALLADIUM-CATALYZED CARBOZINCATION |
1659 |
[10]A. L. J. Beckwith, T. Lawrence, and A. K. Serehis, J. Chem. Soc. Chem. Commun., 1980, 484.
[11] H. Stadtmüller, C. E. Tucker, A. Vaupel, and P. Knochel, Tetrahedron Lett., 1993, 34, 7911.
[12]H. Stadtmüller and P. Knochel, Synlett, 1995, 463.
[13]A. Vaupel and P. Knochel, J. Org. Chem., 1996, 61, 5743.
