

Supplement F2: The Chemistry of Amino, Nitroso, Nitro and Related Groups.
Edited by Saul Patai Copyright 1996 John Wiley & Sons, Ltd.
ISBN: 0-471-95171-4
CHAPTER 3
Chiroptical properties of amino compounds
HOWARD E. SMITH
Department of Chemistry, Vanderbilt University, Nashville, Tennessee 37235, USA Fax: 00 615 322 4936
I. INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
106 |
|
II. ASYMMETRIC SYNTHESIS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
107 |
|
A. Synthesis Using Enzymes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
107 |
|
B. Reduction of Ketoximes and Ketoxime Ethers with Chiral Metal |
|
|
Hydride Reagents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
109 |
|
C. Reduction of Imines with Chiral Metal Hydride Reagents . . . . . . . . . |
112 |
|
D. Reduction of Imines with Hydrogen in the Presence of a Chiral |
|
|
Titanium Hydride Complex . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
114 |
|
E. Amination of Alkenes with Chiral Borohydride Reagents . . . . . . . . . |
117 |
|
F. Aziridination of Alkenes with Chiral Diimine-based Catalysts . . . . . . |
119 |
|
G. Kinetic Resolution Using a Modified Sharpless Reagent . . . . . . . . . . |
120 |
|
III. ESTIMATION OF ENANTIOMERIC EXCESS . . . . . . . . . . . . . . . . . . |
121 |
|
A. Quantitative Aspects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
121 |
|
B. Chromatography Using Chiral Stationary Phases . . . . . . . . . . . . . . . |
121 |
|
C. Nuclear Magnetic Resonance Methods . . . . . . . . . . . . . . . . . . . . . |
124 |
|
IV. ELECTRONIC CIRCULAR DICHROISM (ECD) . . . . . . . . . . . . . . . . |
130 |
|
A. Amino Chromophore . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
130 |
|
B. Effect of the Amino Group on the Benzene Chromophore . . . . . . . . . |
133 |
|
1. |
Benzene sector rule . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
133 |
2. |
Benzene chirality rule . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
135 |
C. Chromophoric Derivatives . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
137 |
|
1. |
N-Salicylidene derivatives and the salicylidenamino chirality rule . . |
137 |
2. |
Exciton coupled circular dichroism (ECCD) spectra . . . . . . . . . . . |
139 |
|
a. N-Benzylidene derivatives . . . . . . . . . . . . . . . . . . . . . . . . . |
139 |
|
b. N-Benzoyl derivatives . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
142 |
D. N-Nitrosamines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
143 |
|
105
106 |
Howard E. Smith |
V. VIBRATIONAL OPTICAL ACTIVITY (VOA) . . . . . . . . . . . . . . . . . . 146 A. Vibrational Circular Dichroism (VCD) . . . . . . . . . . . . . . . . . . . . . 146 B. Raman Optical Activity (ROA) . . . . . . . . . . . . . . . . . . . . . . . . . . 150
VI. REFERENCES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 153
I. INTRODUCTION
In the introduction to the earlier review in 1982, ‘Chiroptical Properties of Amino, Nitroso and Nitro Compounds’1, for which this chapter is a supplement, a brief sketch was given of the historical developments which caused the rotation for sodium D light (589 nm) to be the chiroptical property most frequently reported for chiral substances. Useful compilations of this chiroptical property for natural products, including ˛-amino acids2, alkaloids3 and amino sugars4, are available, and the absolute configurations of a host of chiral substances, including chiral amino, nitroso and nitro compounds, are presented in two collections5,6. In the one which has been recently updated5, the absolute configurations of approximately 9000 compounds, the method by which each configurational assignment was made, the sign of the rotatory power for a particular enantiomer and the appropriate literature reference are given. In the other6, the absolute configurations, the sign of the rotatory power for a given state (liquids or as solutions in various solvents) and literature references are tabulated for 6000 compounds, each compound having only one chiral center (asymmetric carbon atom). Even using these large compilations of rotatory powers, rotatory power comparisons for the establishment of absolute configuration are not as reliable a tool as optical rotatory dispersion7 (ORD) and circular dichroism8 (CD) measurements. Using rotatory power data, however, Brewster9 developed a set of rules which can be used to relate the rotatory power of chiral substances to their absolute configurations, and these rules are sometimes used when other methods of configurational assignment cannot be easily utilized.
The focus of the earlier chapter was a brief outline of the sources of chiral amino compounds, application of Brewster’s rules for the assignment of the absolute configurations to a few chiral amines, a discussion of the ORD and CD in the visible (380 780 nm) and near-ultraviolet (200 380 nm) spectral regions of chiral amino compounds and some of their derivatives, and how the observed Cotton effects (CEs) in their ORD curves and CD spectra relate to their conformational preferences and absolute configurations.
At that time, as now, the enantiomers of many chiral amines were obtained as natural products or by synthesis from naturally occurring amines, ˛-amino acids and alkaloids, while others were only prepared by introduction of an amino group by appropriate reactions into substances from the chiral pool: carbohydrates, hydroxy acids, terpenes and alkaloids. In this connection, a recent review10 outlines the preparation of chiral aziridines from enantiomerically pure starting materials from natural or synthetic sources and the use of these aziridines in stereoselective transformations. Another report11 gives the use of the enantiomers of the ˛-amino acid esters for the asymmetric synthesis of nitrogen heterocyclic compounds.
In synthetic operations, when a symmetrical (achiral) substrate is used, the product is racemic. Diastereomers are separated by physical methods, and the enantiomers of racemic amines are frequently obtained by fractional crystallization of diastereomeric salts formed with chiral acids. Although resolution of racemic amines by fractional crystallization of enantiomeric salts is still an important technique12 and the laboratory scale resolutions of many racemic amines have been reported13, the separation of the enantiomers of chiral amines by chromatography14 16 and their preparation by asymmetric synthesis using enzymes17 and other asymmetric catalysts18 have had extensive development during the

3. Chiroptical properties of amino compounds |
107 |
last decade. These methods are now discussed as important sources of the enantiomers of chiral amines together with recently developed methods for the establishment of enantiomeric excess (ee).
These developments are to be viewed in the light of a trend for the pharmaceutical industry to develop single enantiomeric drugs in the quest for safer, more potent and selective products19 21. However, a more significant factor contributing to the movement toward single enantiomeric drugs is the stance taken by the US FDA (Food and Drug Administration) which in 1992 announced22 the availability of a policy statement, ‘FDA’s Policy Statement for the Development of New Stereoisomeric Drugs’, in connection with the introduction of new drugs. Although the FDA will continue to approve racemates on a case by case basis, companies will be required to demonstrate in their submission documents that the racemic mixture is safe by conducting studies on the individual enantiomers.
In addition to a review of the recent developments in the preparation of chiral amino compounds, developments concerning the interpretation of their ORD and CD in the visible and ultraviolet spectral regions will be reviewed, together with the emerging impact of vibrational (infrared) optical activity (VOA) observations, including vibrational circular dichroism (VCD) and Raman optical activity (ROA) measurements23, on important stereochemical problems concerning chiral amino compounds.
Extensive developments in the preparation of the enantiomers of chiral nitroso and nitro compounds have not appeared since the earlier review1, and thus no additional discussion of the sources of nitroso and nitro compounds is made in this supplement. However, because of the continuing interest in carcinogenic properties of N-nitrosamines and related compounds24, there has been a number of reports concerning the ECD and VCD of such compounds, and the ECD and VCD of nitrosamines are discussed as derivatives of chiral amines.
II.ASYMMETRIC SYNTHESIS
A.Synthesis Using Enzymes
The oldest form of asymmetric synthesis utilized enzymes as catalysts, and very early, enzyme catalysts were applied to the solution of problems associated with the optical resolution of ˛-amino acids and their derivatives, to the subsequent estimation of the optical purity of the isomers so obtained and to the determination of the absolute configurations of specific amino acids25. An example of the usefulness of enzymes for the preparation of pure enantiomers of ˛-amino acids is the stereoselective hydrolysis of synthetic racemic tert-leucinamide hydrochloride (DL-1ÐHCl) in water using hog kidney amidase giving L- tert-leucine (L-2) and leaving D-1 unchanged26. Although this particular enzymic reaction was done to correlate the absolute configurations of the respective enantiomers of 2 with their rotatory powers, the synthesis gave pure samples of D-1 and L-2 in gram quantities. Similar reactions using a commercially available amidase yielding the L-˛-amino acids and the D-˛-amino amides of a number of ˛-amino acids can be followed by synthetic operations which convert the respective enantiomers to the chiral primary amines. Such a sequence of reactions has been reported by which L-phenylalanine (L-3) was converted to
CONH2 |
|
|
CO2 H |
CONH2 |
|
|
|
hog kidney |
C H + |
H C NH2 |
|
CHNH3 Cl |
|
H2 N |
|||
|
|||||
|
C(CH3 )3 |
amidase / H2 O |
C(CH3 )2 |
C(CH3 )3 |
|
|
|
|
|||
(DL-1 HCl) |
|
|
(L-2) |
(D-1) |
|
108 |
Howard E. Smith |
S-2-amino-3-methyl-1-phenylbutane [S-4], the carboxyl group becoming an isopropyl group27. The use of ˛-amino acids and other reagents can afford either enantiomer of a variety of chiral primary amines.
CO2 H |
CH(CH3 )2 |
H2 N C H |
H2 N C H |
CH2 Ph |
CH2 Ph |
(L-3) |
[(S)-4)] |
It is to be noted that in recent years, reviews have extolled the virtues of biocatalysts28, but only a few examples have appeared of the utilization of enzymes as catalysts for the direct formation of the enantiomers of chiral amines in which the amino group is not vicinal to a carboxyl group. One report outlines the resolution of a number of chiral amines by the enzymatic acylation of the amine with trifluoroethyl n-butyrate
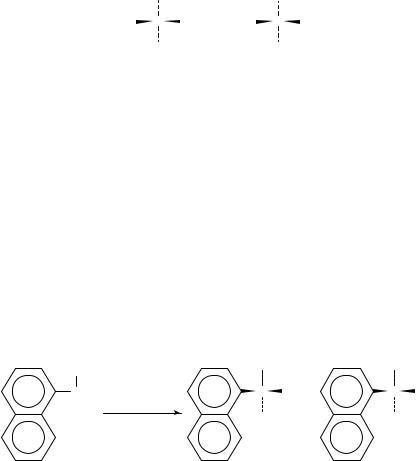
(5) in 3-methyl-3-pentanol, catalyzed by the commercially available enzyme, subtilisin Carlsberg, a protease from Bacillus licheniformis29. For reaction with racemic ˛-(1- naphthyl)ethylamine [(š)-6] with 5 in the presence of subtilisin as the biocatalyst, N- [(S)]-˛-(1-naphthyl)ethyl]butyramide [S-7] was obtained with an ee of 98%. In another experiment with a different set of isolation conditions, the unreacted amine, (R)-˛-(1- naphthyl)ethylamine [R-6], was isolated with an ee of 99%. For the other nine different racemic amines used, the acylation had the same degree of enantioselectivity, and the amide formed had the same generic configuration as S-7. It should be noted that the degree of stereoselectivity for the reaction is extremely sensitive to the solvent used, and no enantioselectivity was observed when toluene, octane or cyclohexane was the solvent29.
CH3 CH2 CH2 CO2 CH2 CF3
|
(5) |
|
|
|
|
CH3 |
|
CH3 |
|
CH3 |
|
|
|
|
|
||
CHNH2 |
|
C H |
|
C NH2 |
|
|
subtilisin / 5 |
NHCOPr |
+ |
H |
|
3-methyl-2-pentanol |
|||||
|
|
|
|||
[(±)-6] |
[(S)-7 (ee 98%)] |
|
[(R)-6 (ee 99%)] |
|
|
A similar enzyme-catalyzed stereoselective synthesis of enantiomers of propanolamines has been recently reported30. Addition of a lipozyme from the fungus Mucor miehei to the epoxide š -8 in toluene and then a slightly more than one half molar equivalent of 2-propylamine gave a 29% conversion of š -8 to S-9 with an ee of 90%. For some benzene ring-substituted epoxides, both the percent conversion of the epoxide and the ee of product are slightly higher30.
Another general method for the preparation of chiral primary amines, which may be less convenient than that using subtilsin Carlsberg, utilizes a microorganism, Bacillus megaterium, Pseudomonas aeruginosa or Pseudomonas putida, grown in such a way that it produces an ω-amino acid transaminase31. An ω-amino acid transaminase is one

|
3. Chiroptical properties of amino compounds |
109 |
|||||
|
O |
OH |
H |
|
|||
|
|
lipozyme / toluene |
|
|
|
|
|
PhO |
|
|
PhO |
|
N |
CH3 |
|
|
|
|
|||||
|
2-propylami ne |
|
|
|
|
||
|
|
|
|
|
|
|
CH3 |
|
[(±)-8] |
|
|
|
[(S)-9 (ee 90%)] |
||
which exhibits the property of converting the terminal CH2 NH2 group of an ω-amino acid to a CHDO group. Thus S-1-phenyl-3-aminobutane [S-10] was deaminated in a racemic mixture š -10 in water in the presence of an ω-amino acid transaminase preparation from Bacillus megaterium, leaving a 60% yield of R-10 with an ee 98% and 4-phenyl-2-butanone (11). Pyruvate anion (12) was the amino group acceptor and was converted to L-alaninate anion (L-13). As shown, the reaction is completely reversible and, using different reaction conditions and product isolation procedures, a ketone such as 11 can be converted to S-10 with the same enzyme and an amino group donor such as L-alaninate (L-13). As an alternate to the formation of the enantiomer of R-10, its chiral center may be inverted by conversion to the N,N-di-(p-toluenesulphonyl)imide [R-14], displacement in dimethylformamide (DMF) of the di(toluenesulphonyl)imide group with azide ion in an SN2 reaction and then reduction of the resulting azide [S-15] with hydrogen over palladium on carbon32. In the reaction shown, the ee of S-10 was found to be the same as that of R-14, since the reaction of R-14 with azide ion goes by way of complete inversion of the configuration at the chiral center in R-1432. The enzymic reaction utilizing an ω-amino acid transaminase is also very general, and an extremely large number of different groups may be attached to the secondary carbon atom to which the amino group is attached. Among these may be alkyl and aryl groups and phenyl groups with a halo, hydroxyl or alkoxyl substituent31.
|
CH3 |
|
CO2− |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CHNH2 |
+ |
C |
|
|
O |
|
|
|
Bacillus magaterium |
|
||
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
||
CH2CH2Ph |
|
CH3 |
|
|
H2 O |
|
|||||||
[(±)-10] |
|
(12) |
|
|
|
|
|
CH3 |
CO2− |
||||
|
|
|
|
|
|
||||||||
|
|
|
|
CH3 |
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
+ H2N C H |
+ H2N C H |
|||
|
|
|
C |
|
O |
||||||||
|
|
|
|
||||||||||
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2CH2Ph |
|
|
CH2CH2Ph |
CH3 |
||||||
|
|
|
(11) |
|
|
|
[(R)-10 (ee 98%)] |
(L-13) |
|||||
B. Reduction of Ketoximes and Ketoxime Ethers with Chiral Metal Hydride Reagents
An early approach to the formation of chiral amines by nonenzymatic asymmetric synthesis was the reduction of prochiral ketoximes and their O-tetrahydropyranyl and O-methyl derivatives with lithium aluminum hydride-3-O-benzyl-1,2-O,O- cyclohexylidene-˛-D-glucofuranose complex (16)33 in ether and prochiral ketoximes

110 |
|
|
|
Howard E. Smith |
|
|
|
|
||||||||
|
CH3 |
|
|
|
|
|
|
|
|
|
|
CH3 |
|
|||
|
|
|
|
|
TsCl/NaH/THF |
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|||||||||
H2 N |
C |
H |
Ts2 N |
C |
H |
|||||||||||
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
CH2 CH2 Ph |
|
|
|
|
|
|
|
|
|
CH2 CH2 Ph |
|||||
[(R)-10 (ee 90%)] |
|
|
|
|
|
|
|
|
[(R)-14 (ee 90%)] |
|||||||
|
|
|
|
|
|
NaN3 |
|
|
|
|
||||||
|
CH3 |
|
|
|
|
DMF |
|
CH3 |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
H2 |
/Pd |
|
C |
|
|
|
|
|||
H |
C |
N3 |
|
|
|
H |
C |
NH2 |
||||||||
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
CH2 CH2 Ph |
|
|
|
|
|
|
|
|
|
CH2 CH2 Ph |
|||||
|
[(S)-15] |
|
|
|
|
|
|
|
|
|
[(S)-10 (ee 90%)] |
|||||
with lithium aluminum hydride-3-O-cyclohexylmethyl-1,2-O,O-cyclohexylidene-˛-D- glucofuranose complex (17)34 in ether. Both aryl alkyl and dialkyl ketoximes and their O-tetrahydropyranyl and O-methyl derivatives were reduced, and afforded amines with the S configuration. Thus, reduction of cyclohexyl methyl ketoxime (18) with 16 gaveS -1-cyclohexylethylamine [ S -19] with an ee of 56%, the highest ee obtained using either 16 or 1733,34 but considered too low to be synthetically useful. The asymmetric reductions with 16 of ten prochiral ketoximes and their O-methyl and O-tetrahydropyranyl derivatives gave amines with the known S configuration. Three additional amines for which the absolute configurations had not been established earlier were, on the basis of a similar enantioselectivity for the reduction, assigned the S configuration33. For reduction with the lithium aluminum hydride glucofuranose complex 17, the degree of asymmetric synthesis was improved as compared to the reduction with 16, but the enantioselectivity was the same34. When the reduction was done with either 16 or 17 in the presence of a small amount of ethanol, the complex was modified by the addition of ethanol, and the reductions gave the amines with the R configuration33,34, a stereochemical outcome consistent with the proposed transition state for the reaction of methyl phenyl ketone with 16 to yield S -˛-phenylethyl alcohol35.
H |
O |
|
|
|
H |
Al |
|
|
H |
O |
|
|
||
|
|
|||
|
H |
|
|
O |
|
|
|
H |
|
|
O |
|
|
|
R |
|
|
O |
|
|
|
|||
|
|
|
||
|
H |
|||
|
|
|||
O
(16)R = C6H5
(17)R = c-C6H11
Substantially higher enantioselectivities were achieved by reduction of the O-alkyl derivatives of prochiral ketoximes with borane (BH3) and chiral auxiliaries prepared
3. Chiroptical properties of amino compounds |
111 |
||
|
16 / ether |
H |
|
CH3 |
CH3 |
|
|
|
|
||
N |
|
NH2 |
|
OH |
|
|
|
(18) |
|
[(S)-19 (ee 56%)] |
|
from ˛-amino acids36. Thus, treatment of the acetophenone O-methyloxime (20) with a twofold excess of borane (BH3) in tetrahydrofuran (THF) and an equivalent amount of the chiral auxiliary S-2-amino-3-methyl-1,1-diphenylbutanol [S-21] gave S-˛- phenylethylamine [S-22] after isolation with an ee of 99%, the chiral auxiliary S-21 prepared from L-valine36. Reduction of the corresponding ketoxime of 20 with borane in the presence of chiral auxiliary S-21 gave S-22 with an ee of only 0.6%36. In reactions using S-21 and borane in THF for the reduction of prochiral ketones, the active reducing agent was shown to have the structure and configuration S-2337, formed by the addition of borane to the oxazaborolidine S-24. For the reduction of prochiral ketones to carbinols, however, a better chiral auxiliary is S-2-(diphenylhydroxymethyl)-pyrrolidine [S-25], synthesized directly by reaction of N-(benzyloxycarbonyl)-L-proline methyl ester with phenylmagnesium chloride in THF37. The chiral auxiliary S-25 has not been used for the reduction of ketoximes or ketoxime ethers.
|
|
|
BH3 (2 eq) / (S)-21 |
|
H |
|
||
|
|
|
CH3 |
THF |
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
NH2 |
|
|
|
|
OCH3 |
|
|
|
|
|
|
(20) |
|
|
|
|
[(S)-22 (ee 99%)] |
||
|
Ph |
|
|
|
Ph |
|
|
Ph |
|
|
|
|
|
|
|
|
|
H |
Ph |
H |
|
Ph |
H |
|
Ph |
|
|
+ |
|
+ |
|
|
|||
|
|
|
H |
O |
|
O |
||
H2 N |
OH |
|
N |
N |
− |
|||
|
|
B |
|
H |
|
|||
|
|
|
H3 B− |
|
B |
|
||
|
|
|
H |
|
|
H |
|
|
|
|
|
|
|
|
|
||
[(S)-21] |
|
|
[(S)-23] |
|
[(S)-24] |
|
||
|
|
|
H |
Ph |
H3C |
Ph |
|
|
|
|
|
|
|
|
|||
|
|
|
|
Ph |
H |
H |
|
|
|
|
|
N |
OH |
H2N |
OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
[(S)-25] |
|
[(1R,2S)-26] |
|
|
||

112 |
Howard E. Smith |
Ring-substituted acetophenone O-methyloximes were reduced to the corresponding ring-substituted analogues of S-˛-phenylethylamines [S-22] with ee values of 71 87%38 using the chiral auxiliary S-21 and borane in THF. This configuration is the same generic configuration as that found on reduction of the E (anti) isomer of ketoxime ethers with borane in the presence of the chiral auxiliary -norephedrine [(1R,2S)-2- amino-1-phenyl-1-propanol; (1R,2S)-26]39. Thus, reduction of the O-methyl derivative of E-phenyl p-tolylmethyl ketoxime [E-27] with borane in THF and the chiral auxiliary (1R,2S)-26 gave a 64% yield of S-1-phenyl-2-(p-tolyl)ethylamine [S-28] with an ee of 92%. Reduction of the Z (syn) isomer [Z-27] gave a similar yield of R-28 also with an ee of 92%. This inverse enantioselectivity between the anti and syn isomers was also observed with other aryl and alkyl ketoxime ethers. The orientation and extent of the asymmetric induction was found to be independent of the bulkiness of the ether group on the nitrogen atom and, as shown in the reaction below, attack of the B H moiety took place from the Re(C) si(N) face of E-27 and the Si(C) si(N) face of Z-27. The si(N) face selection in each case shows that the stereochemistry of the oxime moiety is responsible for the stereoselectivity. Presumably, the stereochemical outcome in the reduction of acetophenone O-methyloxime (20) to S-˛-phenylethylamine [S-22] is the result of 20 existing predominantly in the E configuration.
R1 |
R2 |
|
|
|
BH3 (2 eq) / (1 R,2S)-26 |
N |
|
THF |
|
OCH3 |
|
[(E)-27] R1 = Ph
R2 = p-CH3 C6 H4 CH2 [(Z)-27] R1 = p-CH3 C6 H4 CH2
R2 = Ph
R1 H R2
NH2
[(S)-28] R1 = Ph
R2 = p-CH3 C6 H4 CH2 (ee 92%) [(R)-28] R1 = p-CH3 C6 H4 CH2
R2 = Ph (ee 92%)
In the asymmetric reduction of ketones, stereodifferentiation has been explained in terms of the steric recognition of two substituents on the prochiral carbon by chirally modified reducing agents40. Enantiomeric excesses for the reduction of dialkyl ketones, therefore, are low because of the little differences in the bulkiness of the two alkyl groups40. In the reduction of ketoxime ethers, however, the prochiral carbon atom does not play a central role for the stereoselectivity, and dialkyl ketoxime ethers are reduced in the same enantiomeric excess as are aryl alkyl ketoxime ethers. Reduction of the oxime benzyl ethers of E- and Z-2-octanone with borane in THF and the chiral auxiliary (1R,2S)- 26 gave S- and R-2-aminooctane in 80 and 79% ee, respectively39.
C. Reduction of Imines with Chiral Metal Hydride Reagents
Another early attempt to prepare enantiomers of chiral amines by a nonenzymatic asymmetric synthesis was the reduction of alkyl aryl ketimines and diaryl ketimines using chiral alkoxyl aluminum hydrides, these reducing agents prepared in situ by partial decomposition in ether of lithium aluminum hydride with C -borneol (29), -menthol (30) or-quinine41. Thus reduction of the aryl alkyl ketimines 31 33 with the lithium aluminum hydride- C -borneol (LiAlH4-29) reagent gave the levorotatory enantiomers of the ˛-phenylalkylamines (22, 34 and 35) in very low enantiomeric excess. The absolute configuration of -22 had been previously established as S and, in analogy, the levorotatory isomers of 34 and 35 were also assigned the S absolute configuration. These
3. Chiroptical properties of amino compounds |
113 |
configurational assignments have subsequently been confirmed5. When the lithium aluminum hydride- -menthol (LiAlH4-30) reagent was used, the dextrorotatory amines were obtained, also in low enantiomeric excess, the opposite stereoselectivity for the reducing agents being the result of the opposite configurations, S and R, respectively, for the stereogenic center to which the hydroxyl group is attached in the two alcohols, 29 and 30, from which the reducing reagents were prepared41. When ˛-naphthyl phenyl and phenyl o-tolyl ketimines were reduced with the LiAlH4-29 and LiAlH4-30 reagents, the levorotatory and dextrorotatory isomers, respectively, of the corresponding chiral amines were obtained in low enantiomeric excess, but any conclusions as to the absolute configurations of those amines formed in enantiomeric excess could not be drawn. Reduction of 2,4,6-trimethylphenyl phenyl ketimine with either reagent gave the
corresponding racemic amine41.
HO
HO
(29)
LiAlH4 -29 reagent
R
ether
NH
(31)R = CH3
(32)R = C2H5
(33)R = n-C3H7
(30)
H
R
NH2
[(S)-22] R = CH3 [(S)-34] R = C2H5 [(S)-35] R = n-C3H7
Similar to the reduction of prochiral ketoximes and their O-substituted derivatives33, the reduction of N-(1-phenylethyliden)aniline (36) with a lithium aluminum hydride-3- O-benzyl-1,2-O,O-cyclohexylidene-˛-D-glucofuranose complex (16) gave S -N-phenyl- ˛-phenylethylamine [ S -37] in a rather low enantiomeric excess (24%)42. As in the reduction of ketoximes and ketoxime ethers with this same chiral reagent, the reduction of other N-(1-phenylalkyliden)anilines gave the corresponding chiral amines with enantiomeric excesses too low for practical utility. The reaction, however, was used as a means for the assignment of the S absolute configuration to the levorotatory enantiomers of the N-phenyl-˛-phenyl-n-propylamine and N-phenyl-˛-phenylisopropylamine analogues of S -3742. There is no report of the reduction of prochiral ketimines or N-alkyl or N- phenyl ketimines with borane in THF in the presence of chiral auxiliaries S -21, S -25 or (1R,2S)-26.
In an attempt to prepare alkylamines by asymmetric reduction of imines with chiral hydride reagents, diphenylphosphinyl imines (38), prepared by reaction of ketoximes (39) with chlorodiphenylphosphine [ C6H5 2PCl], were reduced in the presence of a variety of chiral aluminum and boron hydride reagents43. Among the most promising reagents was BINAHL-H44 (40), a chiral hydride compound prepared by the modification of lithium
114 |
Howard E. Smith |
|
||
|
|
16 / ether |
H |
|
CH3 |
|
CH3 |
||
|
|
|
||
N |
|
|
|
NHC6 H5 |
C6 H5 |
|
|
|
|
(36) |
|
|
|
[(S)-37 (ee 24%)] |
aluminum hydride with equimolar amounts of R- or S-1,10 -binaphth-2,20-diol and a simple alcohol, usually methanol or ethanol. Also promising were the chiral hydride reagents (2S,3R)-4-dimethylamino-3-methyl-1,2-diphenyl-2-butanol/LiAlH445 and 9-O- (1,2:5,6-di-O-isopropylidene-˛-D-glucofuranosyl)-9-boratabicyclo[3.3.1]nonane46. Using S-40, the N-diphenylphosphinyl imine 38, prepared from the corresponding ketoxime 39 via the intermediate 41, was reduced to the S-N-(diphenylphosphinyl)amine [S-42] which on hydrolysis afforded S-2-aminobutane [S-43] in rather low yield (38%) but with an enantiomeric excess of 93%43.
CH3CH2 |
CH3 |
|
|
|
CH3CH2 |
|
CH3 |
|
|
(C6 H5)2 PCl |
|
|
|
|
|
|
N |
−40˚C |
|
N |
|||
|
|
|
|
|
|||
|
OH |
|
|
|
|
|
OP(C6H5)2 |
|
(39) |
|
|
|
(41) |
||
CH3CH2 |
H CH3 |
|
(S)-40 / THF |
CH3CH2 |
|
CH3 |
|
|
|
||||||
|
|
||||||
|
|
|
|
|
|
||
|
N |
|
|
|
|
|
N |
H |
P(O)(C6H5)2 |
|
|
P(O)(C6H5) |
|||
[(S)-42) |
|
|
|
|
(38) |
||
1.H+ / H2 O
2.base
CH3CH2 H CH3
NH2
[(S)-43 (ee 93%)]
D. Reduction of Imines with Hydrogen in the Presence of a Chiral Titanium Hydride Complex
More recently, an important chiral titanium catalyst for the asymmetric reduction with hydrogen of N-substituted dialkyl ketimines to enantioenriched amines has been
