
Патфиз1 -13 / 9591550a
.pdf
Journal of Cerebral Blood Flow & Metabolism
24:487–494 © 2004 The International Society for Cerebral Blood Flow and Metabolism Published by Lippincott Williams & Wilkins, Baltimore
Intracerebral Hemorrhage in Mice: Model Characterization and Application for Genetically Modified Mice
*Takehiro Nakamura, *Guohua Xi, *Ya Hua, *†Timothy Schallert, *Julian T. Hoff, and *‡Richard F. Keep
From the Departments of *Neurosurgery and ‡Physiology, University of Michigan, Ann Arbor, Michigan, U.S.A., and the †Department of Psychology and Institute for Neuroscience, University of Texas at Austin, Texas, U.S.A.
Summary: Gene knockout or transgenic animals may assist in elucidating the mechanisms of brain injury after intracerebral hemorrhage (ICH). However, almost all commercially available transgenic or knockout animals are mice. The purpose of this study was to develop an ICH model in mice and to investigate the influence of gender and complement C5 genetic differences on outcome after ICH. Male and female C57BL/6 mice and C5-deficient and -sufficient control mice were anesthetized and then received an injection of 30 L autologous whole blood into the right basal ganglia. Brain water content was studied at 1 and 3 days after ICH. Behavioral tests (forelimb use asymmetry and corner turn test) were performed at 1, 3, 7, 14, 21, or 28 days after ICH. In male mice, brain water content was significantly increased in the ipsilateral basal ganglia 1 and 3 days after ICH, compared with saline injection
controls (P < 0.01). There were marked neurological deficits 1 and 3 days after ICH, with progressive recovery over 28 days. In contrast, although brain edema and behavioral deficits were similar at 1 day after ICH in female and male mice, female mice showed reduced edema at 3 days and a faster recovery of behavioral deficits after ICH. 17 -estradiol treatment in male mice markedly reduced ICH-induced edema (P < 0.01). Brain water content was significantly increased in C5-deficient mice compared with C5-sufficient at 3 days after ICH (P < 0.05). These findings suggest that the mouse ICH model is a reproducible and feasible model. These results also suggest that gender and complement C5 are factors affecting brain injury after ICH. Key Words: Intracerebral hemorrhage—Brain edema—Behavior—Gender—Complement—Mouse.
Hemorrhagic stroke accounts for approximately 15% of all strokes but is associated with a disproportionate degree of morbidity. Many patients with an intracerebral hematoma deteriorate progressively because of secondary edema formation (Kase et al., 1994). Basic science research focusing upon secondary mechanisms of brain injury has produced beneficial information with potential clinical implications for the treatment of intracerebral hemorrhage (ICH). Experimental ICH has been investigated in several models (Andaluz et al., 2002). In these models, the hematoma is usually produced by intracerebral infusion of autologous blood or collagenase to induce vessel rupture. Rats have been the most frequently used species for such studies. In the rat intracerebral blood infusion model, there is reproducible perihematomal brain edema and neurologic deficits (Hua et al.,
Supported by grants NS-17760 (J.T.H.) and NS-39866 (G.X.) from the National Institutes of Health.
Address correspondence and reprint requests to Guohua Xi, Department of Neurosurgery, University of Michigan, 5550 Kresge I, Ann Arbor, MI 48109–0532; e-mail: guohuaxi@umich.edu
2002; Yang et al., 1994), making the model useful for testing potential therapies (Kawai et al., 2002; Kitaoka et al., 2002; Masada et al., 2001; Xi et al., 2001a). Gene knockout or transgenic animals may also assist in elucidating the mechanisms of brain injury after ICH. However, almost all commercially available transgenic and knockout animals are mice, and there are few studies of ICH in that species.
The first aim of this study, therefore, was to develop a reproducible ICH model in the mouse and to delineate changes in brain edema, neurologic outcome, and histology. The second aim was to examine whether brain injury would vary depending upon whether autologous blood or blood from a donor mouse was used. A model using the latter has been described recently (Belayev et al., 2003), and the use of a donor was found to facilitate the experiments. However, evidence has implicated the inflammatory and complement systems in ICH-induced injury (Xi et al., 2001a; Xi et al., 2002), and the induction of these systems may vary with donor versus autologous blood. Next, because combining male and female animals would facilitate studies of genetically
487 |
DOI: 10.1097/01.WCB.0000120788.53851.40 |
488 |
T. NAKAMURA ET AL. |
modified mice, the effects of gender upon ICH-induced brain injury and behavior were examined. Finally, the feasibility of comparing ICH-induced brain injury in complement C5 sufficient and C5 deficient mice was examined using this model. As noted previously, complement has been implicated as being involved in ICH-induced brain injury in a rat model (Hua et al., 2000; Xi et al., 2001a).
MATERIALS AND METHODS
Animal preparation and intracerebral infusion
The University of Michigan Committee on the Use and Care of Animals approved the animal protocols. The experiments used 69 male and 36 female C57BL/6 mice (Charles River Laboratories, Portage, MI, U.S.A.), 8 male C5 deficient (B10.D2/oSnJ) mice and 8 male C5 sufficient (B10.D2/nSnJ) mice (Jackson Laboratories, Bar Harbor, ME, U.S.A.), all approximately 2 months of age. Mice were allowed free access to food and water. The animals were anesthetized with Ketamine (90 mg/kg i.p.) (Abbott Laboratories, Chicago, IL, U.S.A.) and Xylazine (5 mg/kg i.p.) (Lloyd Laboratories, Shenandoah, Iowa, U.S.A.), and the right femoral artery was catheterized by PE10 tube to monitor arterial blood pressure and to sample blood for intracerebral infusion. Blood pH, PaO2, PaCO2, hematocrit, and glucose levels were monitored. Rectal temperature was maintained at 37.5°C using a feedback-controlled heating pad. The mice were positioned in a stereotaxic frame (Model 500, Kopf Instruments, Tujunga, CA, U.S.A.) and a cranial burr hole (1 mm) was drilled near the right coronal suture 2.5 mm lateral to the midline. A 26-gauge needle was inserted stereotaxically into the right basal ganglia (coordinates: 0.2 mm anterior, 3.5 mm ventral, and 2.5 mm lateral to the bregma). Either 30 L autologous whole blood or 30 L saline was infused at a rate of 2 L/min using a microinfusion pump (Harvard Apparatus Inc, South Natick, MA, U.S.A.). The needle was removed, the burr hole was filled with bone wax, and the skin incision was closed with suture after infusion.
Experimental groups
This study was performed in five parts. Part 1 evaluated the ICH mouse model itself, using histological examination, brain water content, and behavioral tests. Male mice received an intracaudate injection of 30 L autologous whole blood or 30L saline. Some animals received a sham operation with needle insertion but no infusion. Six ICH mice were killed 2 hours later for hematoma weight measurement. Some mice (n 28) were killed 1 and 3 days after ICH, and the brains were processed for water content measurement (n 7, per time point). Other animals (n 25) were killed at 3 and 28 days after ICH for histologic examination including hematoma volume measurement (n 5). Behavioral tests were performed at 1, 3, 7, 14, 21, or 28 days after ICH (n 8) or sham operation (n 7).
Part 2 investigated whether the use of donor rather than autologous blood would affect ICH-induced brain edema. For those experiments, five mice received an intracaudate injection of 30 L blood taken from a donor mouse (femoral artery).
Part 3 investigated the influence of gender upon outcome in the mouse ICH model. Animals received an injection of 30 L autologous whole blood or 30 L saline into the right basal ganglia or a sham operation. Brain water content was studied at 1 and 3 days after ICH (n 6, per time point). Behavioral tests were performed at 1, 3, 7, 14, 21, or 28 days after ICH (n 6).
Part 4 examined whether estrogen reduces edema formation after ICH. Five male mice received 17 -estradiol (3.0 mg/kg dissolved in saline plus 1% gelatin) subcutaneously 24 hours before ICH and every 24 hours after ICH (total of four times) for water content study at 3 days after ICH. Five control male mice received vehicle (1% gelatin in saline) injection. All mice were killed 3 days after ICH for brain edema measurement.
Part 5 evaluated whether ICH-induced brain injury differs between complement C5 deficient and sufficient mice. Animals received an injection of 30 L autologous whole blood into the right basal ganglia. Brain water content was studied at 3 days after ICH, and behavior tests were undertaken 1 and 3 days after ICH (n 8).
Brain water and ion contents
Animals were reanesthetized (ketamine 120 mg/kg IP and xylazine 5 mg/kg i.p.) and decapitated. Brains were removed, and a coronal brain slice (approximately 2-mm thick) 2 mm from the frontal pole was cut with a blade. The brain slice was divided into two hemispheres along the midline, and each hemisphere was dissected into the cortex and the basal ganglia. Five samples from each brain were obtained: the ipsilateral and contralateral cortex, the ipsilateral and contralateral basal ganglia, and the cerebellum, which served as a control. Brain samples were immediately weighed on an electric analytical balance (Model AE 100, Mettler Instrument Co, Highstown, NJ, U.S.A.) to obtain the wet weight. Brain samples were then dried at 100°C for 24 hours to obtain the dry weight. Brain water content was determined as: (Wet Weight − Dry Weight)/Wet Weight. The dehydrated samples were digested in 1 mL of 1 mol/L nitric acid for 1 week. The sodium and potassium contents of this solution were measured by flame photometer (model IL 943, Instrumentation Laboratory, Inc. Lexington, MA, U.S.A.). Ion content was expressed in microequivalents per gram of dehydrated brain tissue ( Eq/g dry wt).
Behavioral tests
Two behavioral tests were used: a forelimb use asymmetry test (Schallert et al., 2000) and a corner turn test (Hua et al., 2002; Zhang et al., 2002). The authors have reported the advantages of these tests in a rat ICH model (Hua et al., 2002). Forelimb use during exploratory activity was analyzed in a transparent cylinder (10 cm in diameter and 13 cm in height). The behavior was scored according to the following criteria: independent use of the left or right forelimb for contacting the wall during a full rear to initiate a weight-shifting movement and simultaneous use of both the left and right forelimbs to contact the wall. Behavior was quantified by determining the number of times the ipsilateral (unimpaired) forelimb (I), contralateral forelimb (C), and both forelimbs (B) were used as a percentage of total number of limb usage. A single, overall limb-use asymmetry score was calculated as follows: ForelimbUse Asymmetry Score [I/(I + C + B)] − [C/(I + C + B)].
The second behavioral analysis involved a corner turn test. The mouse was allowed to proceed into a corner, the angle of which was 30°. To exit the corner, the animal could turn either to the left or right. When the mouse turned, its choice of direction was recorded. This was repeated 10 to 15 times, and the percentage of right turns was calculated.
Histology and morphometric analysis
Animals were anesthetized (Ketamine 120 mg/kg IP and Xylazine 5 mg/kg i.p.) before undergoing transcardiac perfusion with 4% paraformaldehyde in 0.1 mol/L PBS (pH 7.4). Removed brains were kept in 4% paraformaldehyde for 12 hours and then immersed in 25% sucrose for 2 to 3 days at 4°C.
J Cereb Blood Flow Metab, Vol. 24, No. 5, 2004
|
INTRACEREBRAL HEMORRHAGE IN MICE |
|
489 |
||||||
TABLE 1. Systemic physiological variables in different strains of mice |
|
|
|||||||
|
|
|
|
|
|
||||
|
C57BL/6-male |
C57BL/6-female |
C5-sufficient-male |
C5-deficient-male |
|||||
|
(n 8) |
(n 6) |
(n 8) |
(n 8) |
|||||
|
|
|
|
|
|
|
|
|
|
pH |
7.38 |
± 0.04 |
7.36 |
± 0.05 |
7.37 |
± 0.03 |
7.39 |
± 0.02 |
|
PCO2 (mmHg) |
41.5 |
± 2.3 |
42.5 |
± 4.0 |
41.6 |
± 2.6 |
41.0 |
± 2.8 |
|
PO2 (mmHg) |
99.9 |
± 27.3 |
101.6 |
± 12.3 |
94.5 |
± 12.9 |
92.6 |
± 7.4 |
|
Hematocrit (%) |
41.1 |
± 1.1 |
41.8 |
± 0.8 |
41.9 |
± 1.4 |
41.6 |
± 1.3 |
|
Glucose (mg/dL) |
97.5 |
± 8.5 |
92.7 |
± 10.8 |
91.3 |
± 8.7 |
89.8 |
± 9.0 |
|
MABP (mmHg) |
74.6 |
± 10.8 |
75.8 |
± 9.7 |
76.3 |
± 10.6 |
68.8 |
± 3.5 |
|
|
|
|
|
|
|
|
|
|
|
Values are mean ± SD. MABP, mean arterial blood pressure.
The brains were embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Inc., Torrance, CA, U.S.A.) and sectioned with a cryostat (16 m thick). Hematoxylin and eosin staining was used for histologic examination. Morphometric analysis was performed by computer-assisted hand delineation of the area of the caudate putamen and ventricle using the NIH image software (Version 1.61, Bethesda, MD, U.S.A.) (Felberg et al., 2002).
Statistical analysis
All data in this study are presented as mean ± SD. Data were analyzed with Student’s t-test or analysis of variance with Scheffé F test. Significance levels were measured at P < 0.05.
RESULTS
Physiologic variables
Systemic physiologic variables are summarized in Table 1. Changes in body weight were calculated 3 days after ICH, and the loss is expressed as a percentage of initial. Male C57BL/6 mice lost significantly more body weight than females (8.8 ± 3.1 vs. 1.2 ± 2.2% loss; P < 0.01). C5-deficient mice also lost significantly more body weight than C5-sufficient mice (10.4 ± 5.4 vs. 6.0
± 4.3%; P < 0.05).
Hematoma weight and volume
Two hours after ICH, average hematoma weight in 6 mice was 8.3 ± 0.7 mg. Three days after intracerebral infusion of 30 l autologous whole blood, hema-
toma volume determined histologically was 5.1 ± 0.3 mm3 (n 5).
Brain atrophy after ICH
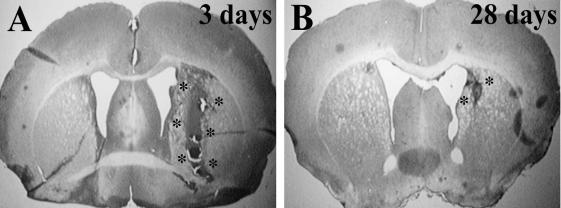
Hematoxylin and eosin staining was performed to examine brain histologic changes 3 and 28 days after ICH in male C57BL/6 mice (Fig. 1). In rats, ICH is associated with delayed brain atrophy and ventricular dilation (Felberg et al., 2002). In the mouse model, there was a tendency for ventricular dilation at 28 days (ipsilateral ventricle 0.46 ± 0.11 mm2 vs. 0.33 ± 0.08 mm2 in contralateral ventricle), but this did not reach significance (P 0.06). Similarly, the area of the ipsilateral caudate putamen (3.94 ± 0.44 mm2) was also not significantly reduced compared with contralateral (4.10 ± 0.45 mm2; P 0.58).
Brain water and ion contents
Compared with saline-injected controls, the brain water content was significantly increased in the ipsilateral basal ganglia and cortex in male C57BL/6 mice 1 and 3 days after injection of autologous blood (P < 0.01) (Fig. 2). ICH-induced brain edema formation after ICH was associated with an accumulation of sodium in the ipsilateral basal ganglia (1 day: 256 ± 35 vs. 153 ± 20 Eq/g dry weight in controls, P < 0.01; 3 days: 254 ± 31 vs. 146
± 19 Eq/g dry weight, P < 0.01) and a loss of potassium
FIG. 1. Hematoxylin and eosin stained brain coronal sections 3 days (A) and 28 days (B) after infusion of 30 µL autologous blood into male C57BL/6 mice. *hematoma location.
J Cereb Blood Flow Metab, Vol. 24, No. 5, 2004
490 |
T. NAKAMURA ET AL. |
Influence of gender
Although the degree of brain edema in the ipsilateral basal ganglia was not significantly different in female and male C57BL/6 mice 1 day after ICH, female mice had significantly less brain edema at 3 days (Fig. 5A). The sodium and potassium content in ipsilateral basal ganglia was also significantly different between male and female C57BL/6 mice 3 days after ICH (Figs. 5B and 5C). Systemic administration of 17 -estradiol in male C57BL/6 mice significantly reduced brain water content in the ipsilateral cortex and basal ganglia 3 days after ICH (Fig. 6). Evidence for a faster recovery from brain injury was also found in behavioral testing. Although there was no significant difference in behavioral
FIG. 2. Brain water content 24 (A) and 72 (B) hours after infusion of 30 µL autologous blood or 30 µL saline into male C57BL/6 mice. Values are expressed as mean ± SD; n = 7 in each group. *P < 0.01 compared with saline injection group. Cont-CX, contralateral cortex; Ipsi-CX, ipsilateral cortex; Cont-BG, contralateral basal ganglia; Ipsi-BG, ipsilateral basal ganglia; Cerebel, cerebellum.
(3 days: 317 ± 15 vs. 368 ± 20 Eq/g dry weight in controls, P < 0.01).
Behavioral tests
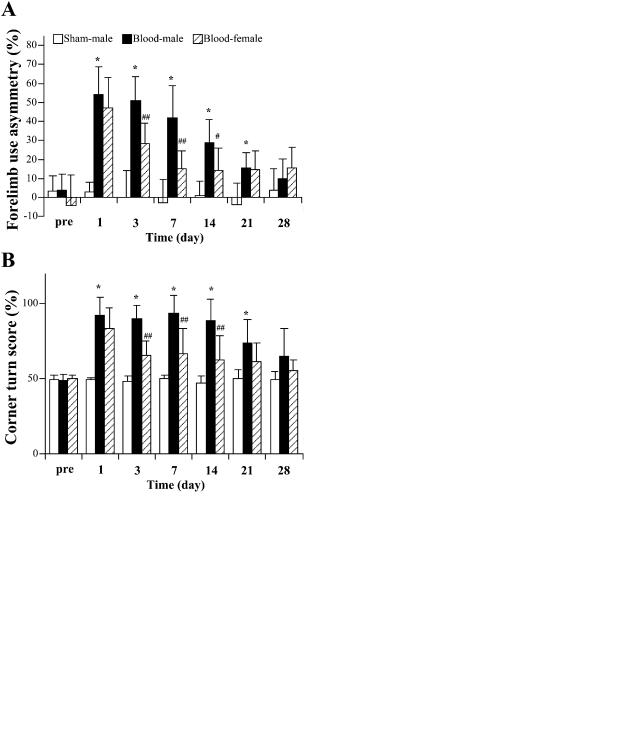
Within one day of injection of autologous blood into the brain of male C57BL/6 mice, there were marked behavioral deficits as assessed by forelimb use asymmetry and corner turn tests (Fig. 3). However, both tests demonstrated a gradual recovery of function so that there was no significant behavioral deficit compared with sham-operated animals 28 days after blood injection (Fig. 3).
Autologous versus donor blood
In the case of donor blood injection, brain water content was significantly increased in the ipsilateral basal ganglia in male mice 1 day after ICH, compared with that of male mice that received an autologous blood injection (P < 0.05) (Fig. 4).
FIG. 3. The time course of behavioral deficits after ICH in male and female C57BL/6 mice. Forelimb use asymmetry (A) and corner turn test (B) scores were measured before ICH and at 1, 3, 7, 14, 21, and 28 days after 30 µL autologous blood infusion (in male and female mice) or in sham controls (needle insertion without infusion) in male mice. Values are expressed as mean ± SD; n = 8 in blood injection male group, n = 6 in blood injection female group, and n = 7 in sham control group. *P < 0.01 compared with sham-operated group; #P < 0.05 and ##P < 0.01 between male and female ICH mice.
J Cereb Blood Flow Metab, Vol. 24, No. 5, 2004

INTRACEREBRAL HEMORRHAGE IN MICE |
491 |
DISCUSSION
ICH in mice
There are few reports of ICH models in mice. Clark and colleagues (1998) reported an ICH model induced by collagenase injection into the caudate nucleus, and, very recently, Belayev and colleagues (2003) have established a mouse ICH model induced by infusion of 15 L donor blood into the striatum. To our knowledge, the present study is the first report of an intracerebral autologous blood infusion model in mice. In this model, 30 L of
FIG. 4. Brain water content in male C57BL/6 mice 1 day after infusion of 30 µL of either autologous or donor blood. Values are expressed as mean ± SD; n = 5 in each group. *P < 0.05 between autologous and donor blood groups. Cont-BG, contralateral basal ganglia; Ipsi-BG, ipsilateral basal ganglia.
deficit between male and female mice at 1 day after ICH as assessed by the forelimb use asymmetry score or the corner turn test score, both tests detected a quicker recovery in female mice (Fig 3). Thus, in both tests, the female mice did significantly better at 3, 7, and 14 days after ICH. By 21 and 28 days after ICH, the male mice had recovered to levels that were not significantly different from the female mice.
Influence of genetic difference of complement C5
One C5 deficient mouse died approximately 6 hours after intracerebral blood infusion. Other C5-deficient (n 7) and C5-sufficient (n 8) mice survived until the water content study at 3 days after injection, although, as noted previously, the C5-deficient mice lost more weight. Brain water content in the ipsilateral basal ganglia 3 days after ICH was significantly increased in C5-deficient mice compared with C5-sufficient mice (82.0 ± 1.0 vs. 80.5 ± 1.0%, P < 0.05) (Fig. 7). The increased edema in the C5-deficient mice was associated with a significantly (P < 0.05) greater accumulation of sodium and loss of potassium than in C5-sufficient mice (Na+: 260 ± 9 vs. 238 ± 4 Eq/g dry weight; K+: 305 ± 5 vs. 338 ± 10 Eq/g dry weight). There was no significant difference in water or ion content in the contralateral basal ganglia between C5-deficient and C5-sufficient mice. For behavioral testing, there was no significant difference in corner turn score 1 day after ICH, but the C5-deficient mice were significantly more impaired at day 3 (91.4 ± 8.9 vs. 80.0 ± 10.7% in C5 sufficient mice, P < 0.05).
FIG. 5. Brain water (A), sodium (B), and potassium (C) in male and female C57BL/6 mice 1 and 3 days after infusion of 30 µL autologous blood. Values are expressed as mean ± SD; n = 7 in males and n = 6 in females. *P < 0.01 between male and female mice. Cont-BG, contralateral basal ganglia; Ipsi-BG, ipsilateral basal ganglia.
J Cereb Blood Flow Metab, Vol. 24, No. 5, 2004

492 |
T. NAKAMURA ET AL. |
FIG. 6. The effect of 17 -estradiol treatment on brain water content 3 days after infusion of 30 µL autologous blood. Values are expressed as mean ± SD; n = 5 in vehicle group and n = 5 in 17 -estradiol treatment group. *P < 0.05, **P < 0.01 compared with saline injection group at the and levels, respectively. ContCX, contralateral cortex; Ipsi-CX, ipsilateral cortex; Cont-BG, contralateral basal ganglia; Ipsi-BG, ipsilateral basal ganglia; Cerebel, cerebellum.
blood was infused because a 30- L hematoma in a mouse corresponds to an average-sized intracerebral hematoma in a human (approximately 60 mL) (Volpin et al, 1984; Xi et al, 2001a). The infusion of this amount of blood induced marked and reproducible brain edema and neurologic deficits. The question of whether donor blood (as used by Belayev et al., 2003) could be used instead of autologous blood was examined. Donor blood induced significantly more brain edema than autologous blood. Evidence indicates that complement system activation and inflammation are both involved in brain injury (Xi et al., 2001a), and it is possible that the use of donor blood may amplify these events.
Brain edema and neurologic deficits are important clinical endpoints for ICH studies. Rats have been the
FIG. 7. Brain water content in complement C5 sufficient and deficient mice 3 days after infusion of 30 µL autologous blood. Values are expressed as mean ± SD; n = 8 in C5-sufficient group and n = 7 in C5-deficient group. *P < 0.05 compared with the saline injection group. Cont-CX, contralateral cortex; Ipsi-CX, ipsilateral cortex; Cont-BG, contralateral basal ganglia; Ipsi-BG, ipsilateral basal ganglia; Cerebel, cerebellum.
most frequently used species for ICH studies and intracerebral infusion of blood (50 or 100 L) produces reproducible brain edema and neurologic deficits in that species (Andaluz et al., 2002). A number of potential treatment regimens have been shown to improve outcome in rats (Kawai et al., 2002; Kitaoka et al., 2002; Masada et al., 2001; Xi et al., 2001a,b). The mouse ICH model produces very similar behavioral deficits to those found in the rat (Hua et al., 2002); indeed, the time course of behavioral recovery is also very similar between the two species. In both rat and mouse models, there is no significant difference between ICH and shamoperated animals at 21 days after ICH in both forelimb use asymmetry and corner turn tests (Hua et al., 2002). The amount of brain edema measured in the mouse model at 1 and 3 days (80%) is somewhat less than that found in the rat (81.5% at 1 day and 83.5% at 3 days) (Xi et al., 1998). It is still approximately 3% greater than saline-injected controls: this suggests that it should still be possible to examine agents that reduce edema formation in the mouse model. Perhaps related to the reduced edema formation in the mouse, mice did not display the marked ventricular dilation and caudate putamen atrophy found in the rat (Felberg et al., 2002). There was some slight evidence of ventricular dilation at 28 days in the mouse, and it is possible that brain atrophy will develop later in the mouse.
Influence of gender
Although the initial brain injury after ICH, as assessed by brain edema and behavioral deficits, appeared similar between female and male mice, both of these measurements indicated a faster recovery after injury in the female mice. Previously, reduced susceptibility to ischemic and traumatic brain injury has been reported in females in experimental models. In ischemic stroke models, brain infarcts are significantly reduced in females than in males (Alkayed et al., 1998; Zhang et al., 1998). In traumatic brain injury, brain edema is significantly less in female compared with male rats (Roof et al., 1993a). Moreover, Roof and colleagues (1993b) showed that whereas male rats were significantly impaired in their performance on behavioral tasks after cortical injury, lesioned females performed the task as well as noninjured controls. In a mouse experimental head injury model, Kupina and colleagues (2003) showed that cytoskeletal protein degradation and neurodegeneration evolve differently in males and females. The greater neuroprotection afforded to females in cerebral ischemia and trauma is likely because of the effects of circulating estrogens and progestins (Roof and Hall., 2000). Our data on the effect of 17 -estradiol on ICH-induced edema in males also support a role for estrogen in reducing hem- orrhage-induced brain injury.
J Cereb Blood Flow Metab, Vol. 24, No. 5, 2004
INTRACEREBRAL HEMORRHAGE IN MICE |
493 |
In ICH, much attention has focused upon the role of the coagulation cascadeand iron-induced brain injury (Xi et al., 2001b). There is little information about how these two processes would be affected by gender. Of interest is the finding by Culmsee and colleagues (1999) that estrogen protects against iron-induced neurotoxicity in vitro. Systemically there are differences in the coagulation cascade and iron handling between males and females (Kapiotis et al., 1998; Nanji et al., 2001). The underlying basis for gender differences in ICH-induced injury requires further systematic investigation. It should be noted that our results indicate that a combination of males and females should not be used in same ICH experiment even with genetically modified mice of limited availability.
Application for genetically modified mice
Complement activation in the brain has been found in a variety of central nervous system diseases (Morgan et al., 1997), including a rat ICH model (Hua et al., 2000). Studies have indicated that such complement activation may be involved in inducing brain injury in hemorrhagic (Hua et al., 2000) and ischemic (Vasthare et al., 1998) brain damage. However, there have been some studies indicating that elements of the complement system may have beneficial effects (Mukherjee and Pasinetti, 2000). Thus, for example, Pasinetti and colleagues (1996) reported increased kainic acid-induced hippocampal damage in C5 deficient mice. Similarly, the current study found increased ICH-induced brain edema formation and behavioral deficits in C5-deficient mice. Pasinetti and colleagues (1996) also found increased lipopolysaccha- ride-induced production of interleukin-6 and tumor necrosis factor- (TNF- ) from astrocytes derived from C5-deficient mice. It is interesting that our previous studies have shown that perihematomal TNFcon- centrations are increased 2 hours after ICH in rats (Xi et al., 2001a).
The development of the mouse ICH model should enable dissection of which elements of the complement system have beneficial and detrimental effects in ICH. However, a word of caution is necessary. The effects of an element of the complement system may depend on the absolute level of activity, that is, partial inhibition may have different effects from total deficiency. This would be analogous to the situation with heme oxygenase 1 in brain injury, which may be protective or detrimental depending upon concentration (Gonzales et al., 2002). (Mukherjee and Pasinetti, 2000)
CONCLUSION
The results of the present study indicate that the autologous blood infusion ICH model can be adapted to the mouse. This mouse model is suited to further studies on influence of genotype on ICH outcomes. In addition,
future studies should examine whether use of estrogen is a useful treatment for ICH in humans.
REFERENCES
Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD (1998) Gender-linked brain injury in experimental stroke. Stroke 29:159–165
Andaluz N, Zuccarello M, Wagner KR (2002) Experimental animal models of intracerebral hemorrhage. Neurosurg Clin N Am 13: 385–393
Belayev L, Saul I, Cubelo K, Busto R, Belayev A, Zhang Y, Riyamongkol P, Zhao W, Ginsberg MD (2003) Experimental intracerebral hemorrhage in the mouse. Histological, behavioral, and hemodynamic characterization of a double-injection model. Stroke 34:2221–2227
Clark W, Gunion-Rinker L, Lessov N, Hazel K (1998) Citicoline treatment for experimental intracerebral hemorrhage in mice. Stroke 29:2136–2140
Culmsee C, Vedder H, Ravati A, Junker V, Otto D, Ahlemeyer B, Krieg JC, Krieglstein J (1999) Neuroprotection by estrogens in a mouse model of focal cerebral ischemia and in cultured neurons: evidence for a receptor-independent antioxidative mechanism.
J Cereb Blood Flow Metab 19:1263–1269
Felberg RA, Grotta JC, Shizadi AL, Strong R, Narayana P, Hill-Felberg SJ, Aronowski J (2002) Cell death in experimental intracerebral hemorrhage: The “black hole” model of hemorrhagic damage. Ann Neurol 51:517–524
Gonzales S, Erario MA, Tomaro ML (2002) Heme oxygenase-1 induction and dependent increase in ferritin. A protective antioxidant stratagem in hemin-treated rat brain. Dev Neurosci 24:161–168
Hua Y, Xi G, Keep RF, Hoff JT (2000) Complement activation in the brain after experimental intracerebral hemorrhage. J Neurosurg 92: 1016–1022
Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G (2002) Behavioral tests after intracerebral hemorrhage in the rat. Stroke 33:2478– 2484
Kapiotis S, Jilma B, Pernerstorfer T, Stohlawetz P, Eichler HG, Speiser W (1998) Plasma levels of activated factor VII decrease during the menstrual cycle. Thromb Haemost 80:588–591
Kase CS, Caplan LR (1994) Intracerebral Hemorrhage. Boston: But- terworth-Heinemann
Kawai N, Nakamura T, Nagao S (2002) Effects of brain hypothermia on brain edema formation after intracerebral hemorrhage in rats. Acta Neurochir (Suppl 81):233–235
Kitaoka T, Hua Y, Xi G, Hoff JT, Keep RF (2002) Delayed argatroban treatment reduces edema in a rat model of intracerebral hemorrhage. Stroke 33:3012–3018
Kupina NC, Detloff MR, Bobrowski WF, Snyder BJ, Hall ED (2003) Cytoskeletal protein degradation and neurodegeneration evolves differently in males and females following experimental head injury. Exp Neurol 180:55–73
Masada T, Hua Y, Xi G, Yang GY, Hoff JT, Keep RF (2001) Attenuation of intracerebral hemorrhage and thrombin-induced brain edema by overexpression of interleukin-1 receptor antagonist.
J Neurosurg 95:680–686
Morgan BP, Gasque P, Singhrao S, Piddlesden SJ (1997) The role of complement in disorders of the nervous system. Immunopharmacology 38:43–50
Mukherjee P, Pasinetti GM (2000) The role of complement anaphylatoxin C5a in neurodegeneration: implications in Alzheimer’s disease. J Neuroimmunol 105:124–130
Nanji AA, Jokelainen K, Fotouhinia M, Rahemtulla A, Thomas P, Tipoe GL, Su GL, Dannenberg AJ (2001) Increased severity of alcoholic liver injury in female rats: role of oxidative stress, endotoxin, and chemokines. Am J Physiol Gastrointest Liver Physiol
281:1348–1356
Pasinetti GM, Tocco G, Sakhi S, Musleh WD, DeSimoni MG, Mascarucci P, Schreiber S, Baudry M, Finch CE (1996) Hereditary deficiencies in complement C5 are associated with intensified neurodegenerative responses that implicate new roles for C-system in neuronal and astrocytic functions. Neurobiol Dis 3:197–204
J Cereb Blood Flow Metab, Vol. 24, No. 5, 2004
494 |
T. NAKAMURA ET AL. |
Roof RL, Duvdevani R, Stein DG (1993a) Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res 607:333–336
Roof RL, Hall ED (2000) Gender differences in acute CNS trauma and stroke: Neuroprotective effects of estrogen and progesterone.
J Neurotrauma 17:367–388
Roof RL, Zhang Q, Glasier MM, Stein DG (1993b) Gender-specific impairment on Morris water maze task after entorhinal cortex lesion. Behav Brain Res 57:47–51
Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. (2000) CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacol 39:777–787
Vasthare US, Barone FC, Sarau HM, Rosenwasser RH, DiMartino M, Young WF, Tuma RF (1998) Complement depletion improves neurological function in cerebral ischemia. Brain Res Bull 45:413–419 Volpin L, Cervellini P, Colombo F, Zanusso M, Benedetti A (1984) Spontaneous intracerebral hematomas: a new proposal about the usefulness and limits of surgical treatment. Neurosurgery 15:663–666 Xi G, Keep RF, Hoff JT (1998) Erythrocytes and delayed brain edema
formation following intracerebral hemorrhage in rats. J Neurosurg 89:991–996
Xi G, Hua Y, Keep RF, Younger JG, Hoff JT (2001a) Systemic complement depletion diminishes perihematomal brain edema in rats. Stroke 32:162–167
Xi G, Hua Y, Bhasin RR, Ennis SR, Keep RF, Hoff JT (2001b) Mechanisms of edema formation after intracerebral hemorrhage: effects of extravasated red blood cells on blood flow and blood-brain barrier integrity. Stroke 32:2932–2938
Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT (1994) Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats.
J Neurosurg 81:93–102
Zhang L, Schallert T, Zheng GZ, Jiang Q, Arniego P, Li Q, Lu M, Chopp M (2002) A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods 117:207–214
Zhang YQ, Shi J, Rajakumar G, Day AL, Simpkins JW (1998) Effects of gender and estradiol treatment on focal brain ischemia. Brain Res 784:321–324
J Cereb Blood Flow Metab, Vol. 24, No. 5, 2004
