
1,4-Diformyl-2,3,5,6-TetranitratopiperazineA New Primary Explosive Based on Glyoxal
.pdf
Propellants, Explosives, Pyrotechnics 28 (2003), No. 1 |
1 |
1,4-Diformyl-2,3,5,6-Tetranitratopiperazine: A New Primary Explosive Based on Glyoxal
Konstantin Karaghiosoff, Thomas M. Klapˆtke*, Alexej Michailovski, Heinrich Nˆth, and Max Suter
Department of Chemistry, Ludwig-Maximilians-Universit‰t M¸nchen, Butenandtstr. 5 ± 13 (Haus D), D-81377 M¸nchen (Germany)
Gerhard Holl
Wehrwissenschaftliches Institut f¸r Werk-, Explosivund Betriebsstoffe, Grosses Cent, D-53913 Swisttal (Germany)
Abstract |
Equally important, most solid-propellant driven rockets |
||||
|
use a mixture based on ammonium perchlorate with |
||||
In the present work we describe synthesis and properties of |
aluminum and binding polymers. The exhaust plumes of |
||||
1,4-diformyl-2,3,5,6-tetranitratopiperazine. The 1,4-diformyl-2,3,5,6- |
such rockets contain |
high amounts of |
HCl |
and solid |
|
tetranitratopiperazine turned out to be a powerful highly ener- |
|||||
aluminum oxides [1] |
which, additionally |
to |
the danger |
||
getic compound. Its physical and chemical properties were |
|||||
they pose to the environment, strongly magnify the radar |
|||||
investigated by theoretical as well as experimental methods. The |
|||||
detonation energy of 1,4-diformyl-2,3,5,6-tetranitratopiperazine |
profile of the rocket. |
|
|
|
|
was determined to 5376 kJ/kg or 93% compared to HMX. |
The goal of our work is to find new compounds, which are |
||||
Molecular and crystal structure of 1,4-diformyl-2,3,5,6-tetranitra- |
free of halogens and |
heavy metals and can |
be used to |
||
topiperazine was elucidated by single crystal X-ray diffraction |
|||||
substitute the existing initiators or solid rocket propellants. A |
|||||
analysis. An interesting feature of the title substance, its rotamer |
|||||
equilibrium, was investigated by 2D NMR methods. |
series of substances which contain both fuel and enough |
||||
Keywords: Tetranitratopiperazine, 2-D NMR Spectroscopy, |
oxidizer to produce gaseous products are the 2,3,5,6-tetrani- |
||||
tratopiperazines. The most promising member of the series, |
|||||
Glyoxal, Primary Explosive, Bomb Calorimetry |
|||||
the 1,4-diformyl derivative, is presented in this work. |
|||||
|
|||||
1 Introduction |
2 Synthesis |
|
|
|
|
Since toxic lead and silver azides are still widely used as initiators in ammunition [1], there is an obvious demand for nontoxic substitutes which can be used as primary detonators. During the last years, increased efforts were made towards substitution of heavy metal containing primary detonators through metal free substances. Significant successes are reported in the area of organic azides [2 ± 4] and especially mixed azide-nitrates [5].
(Caution: Compound (1) is highly sensitive to shock and impact. Appropriate safety precautions should be taken all time.)
1,4-Diformyl-2,3,5,6-tetranitratopiperazine (1): 1,4-di- formyl-2,3,5,6-tetrahydroxypiperazine [6] (10 g; 0.048 mol) was added to a solution of 100% nitric acid (9 ml; 13.68 g; 0.217 mol) (Fluka) in trifluoroacetic anhydride (50 ml) (Fluka) under stirring and ice/salt bath cooling. The reaction
Figure 1. Synthesis of 1,4-diformyl-2,3,5,6-tetranitratopiperazine.
* Corresponding author; e-mail: tmk@cup.uni-muenchen.de
¹ 2003 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim |
0721-3113/03/2801/0001 $ 17.50+.50/0 |

2 |
K. Karaghiosoff, T. M. Klapˆtke, A. Michailovski, H. Nˆth, M. Suter and G. Holl |
mixture was warmed up to ambient temperature with stirring, the colorless precipitate that had been formed was separated, washed with 50 ml of water, 50 ml of ethanol and 100 ml of diethyl ether and dried in air. The crude product was recrystallized from 60 ml of acetone. After cooling to20 C 40 ml of diethyl ether were added in order to complete the crystallization.
Adding a small amount of diethyl ether to an acetonic solution of (1) and cooling the solution to 20 C for 5 d yielded crystals suitable for X-ray diffraction.
2.1 Results of Characterization of (1)
Yield: 17.6 g (0.046 mol; 95%) Mp 130 ± 134 C (Dec.)
Raman (1064 nm, 200 mW, in cm 1): 199 (s), 234 (s), 299 (s), 326 (s), 439 (s), 480 (s), 588 (s), 623 (s), 659 (s), 854 (s), 1007 (vs), 1286 (vs), 1411 (s), 1647 (s, N ± O), 1732 (vs, N ± O), 2951 (m, CH), 3013 (s, CH)
1H-NMR (D6 acetone), $7.20 (s, CHONO2, anti-(1)), 7.26 (s, CHONO2, syn-(1)), 7.38 (s, CHONO2, syn-(1)), 7.43 (s, CHONO2, anti-(1)), (sum 4H), 8.80 (s, 2H, N-CHO);
13C{ 1H} -NMR (D6 acetone), $74.0 (CHONO2, syn-
(1)), 74.3 (CHONO2, anti-(1)), 80.5 (CHONO2, synand anti-(1)), 162.6 (N-CHO)
15N-NMR (D6 acetone), $262 (ring N, 1/2 $1200 Hz), 54 (ONO2, 1/2 $136 Hz)
C6H6N6O14 (386.1): found N 21.7%, C 18.8%, H 2.0% (Theory N 21.8%, C 18.7%, H 1.6%)
Table 1. Haber-Born cycle for (1).
Reaction |
|
RH |
|
|
(kJ mol 1) |
C6H6N6O14(s) #1/2O2(g) 3N2(g) #3H2O(l) #6CO2(g) (1) |
2348 |
|
CO(g) #1/2O2(g) CO2(g) |
(2) |
286 |
C6H6N6O14(s) 3N2(g) #3H2O(l) #5CO2(g) #CO(g) |
(3) |
2062 |
under 3 MPa oxygen pressure. The combustion energy was measured to be 2347 kJ mol 1. Following the Haber-Born cycle (Table 1), the detonation energy of (1) can be calculated to 2059 kJ mol 1 or 5339 kJ kg 1:
For comparison, the detonation energy amounts to
797 kJ kg 1 for TNT or 1142 kJ kg 1 for tetryl.
Using the experimentally obtained reaction enthalpy (3 in
Table 1) of RH $ 2059 kJ mol 1 and the standard heats of formation [10] of CO(g) ( 110.5 kJ mol 1), CO2(g) ( 393.7 kJ mol 1), H2O(l) ( 285.8 kJ mol 1), O2(g) (0 kJ mol 1) and N2(g) (0 kJ mol 1), the enthalpy of formation of
(1) can be given as:
fH (1),s $ 874 kJ mol 1
The decomposition of compound (1) is significantly more exothermic compared with TNT or HMX [11]; TNT is a slightly exothermic substance with fH (TNT) $66.9 kJ mol 1, while HMX is strongly endothermic withfH (HMX) $#253.6 kJ mol 1.
3 Explosive Properties
A portion of 40 mg of (1) was subjected to a drop-hammer test using a 5 kg weight dropped from 50 cm height [7]. The substance detonated, yielding a sonic sound intensity of 152 dB. As a comparison, on detonation of the same amount of lead azide, 147 dB are measured. The detonation energy of lead azide amounts to 1536 kJ/kg [8]. From the sound intensity difference of 5 dB the detonation energy ratio of
(1) and lead azide can be calculated to 25/3 $3.17, as the energy doubles with every 3 dB increase in sound intensity. The detonation energy of (1) can be estimated to 4866 kJ/kg, provided that the same fraction of detonation energy is released as sonic energy by both substances.
A small portion of (1) was subjected to rapid heating to 400 C. A violent decomposition with strong gas and light development was observed, however the substance did not detonate.
During slow heating of a very small portion of (1) in a melting point determination apparatus, a fast decomposition and gas development was observed at the melting point.
4 Calorimetry
Five portions of (1) were burned in a Parr 1356 bomb calorimeter [9], equipped with a Parr 207A oxygen bomb,
5 Computational Aspects
The molecular structure and energy parameters of (1) were calculated using the software package Gaussian98 [12]. The calculations were carried out on the density-functional theory level B3PW91 with the basis set 6-311G(d). The hybrid method B3PW91 makes use of parameter exchange functionals of Becke [13], the non-local correlation is determined by the Perdew91 functional [14, 15]. The structure was optimised within C1 symmetry group. The fully optimized structure of 1,4-diformyl-2,3,5,6-tetranitra- topiperazine agrees well with the experimentally determined structure in the solid state.
The heat of detonation according to Eq. (1) was calculated using the DFT energies from Table 2 ( E(4) $1724 kJ mol 1), which after zero point energy correction ( zpeB3PW91(4) $ 134.3 kJ mol 1) and corrections for the translational ( UTr(4) $33/2 ¥ R ¥ T) and rotational term ( URot(4) $12 ¥ R ¥ T), could be converted into the detonation enthalpy 1929 kJ mol 1 or 4996 kJ kg 1 at 298 K:
RH (4) $ 1929 kJ mol 1;
C6H6N6O14(g) 3N2(g) #3H2O(g) #5CO2(g) #CO(g) |
(1) |
With an approximated heat of sublimation of subH (1) of 150.2 kJ mol 1 (this value was taken to be similar to the sublimation enthalpy of PETN [16]) and evaporation
Propellants, Explosives, Pyrotechnics 28 (2003), No. 1
1,4-Diformyl-2,3,5,6-Tetranitratopiperazine
Table 2. Computational results (B3PW91/6-311G(d)).
Compound |
|
E / a.u. |
zpe / kJ mol 1 |
|
|||
CO |
113.209 |
2.96 |
|
CO2 |
188.425 |
6.79 |
|
H2O |
|
76.358 |
13.0 |
N2 |
109.423 |
3.39 |
|
(1) |
1612.020 |
118.2 |
|
3
enthalpy of water [10] of 43.9 kJ mol 1 , the detonation enthalpy of (1) can be given as:
DetH (5) $ 1912 kJ mol 1;
C6H6N6O14(s) 3N2(g) #3H2O(l) #5CO2(g) #CO(g) |
(2) |
This value is in a fairly good accord with the experimentallydetermineddetonationenthalpyvalueof 2059 kJmol 1.
6 NMR Spectroscopy
The 1H-NMR spectrum of (1) displays for the methine protons four signals at $7.20, 7.26, 7.38 and 7.43 ppm; for the methine carbon atoms three signals at $74.0, 74.3 and 80.5 ppm are observed. The spectra indicate the presence of a mixture of two rotamers (syn-(1) and anti-(1)) in solution.
Figure 2. Molecular structure of 1,4-diformyl-2,3,5,6-tetranitra- topiperazine fully optimized at B3PW91/6-311G(d) level of theory.
Scheme 1. Synand anti-Conformer of 1,4-Diformyl-2,3,5,6- tetranitratopiperazine (1).
Figure 3. 1H,1H COSY45 NMR spectrum of (1), 0.1 M solution in D6 acetone, signals of the ring methine protons.
Propellants, Explosives, Pyrotechnics 28 (2003), No. 1

4 |
K. Karaghiosoff, T. M. Klapˆtke, A. Michailovski, H. Nˆth, M. Suter and G. Holl |
Figure 4. 13C, 1H HETCOR NMR spectrum of (1), 0.1 M solution in D6 acetone, signals of the ring methine protons.
The interconversion between the rotamers is slow on the NMR time scale.
The assignment of the 1H-NMR signals of the ring protons to the single rotamers results from the 1H-COSY45 NMR spectrum (see Fig. 3). It clearly shows cross peaks between the signals at $7.26 and 7.38, originating from H,H- coupling over three bonds between the anisochronous vicinal ring protons in anti-(1). The other two signals at$7.20 and 7.43 are consequently attributed to the ring methine protons in syn-(1). Assignment of the 13C-NMR signals of the ring carbon atoms is based on a 1H, 13C HETCOR NMR spectrum, the methine region of which is shown in Fig. 4. Interestingly, the 13C-NMR signals of one methine group of each syn-(1) and anti-(1) overlap at $ 80.5, resulting in three (instead of the expected four) observed signals in the one dimensional 13C-NMR spectrum.
Data Collection and Processing. Siemens P4 instrument
equipped with a CCD Area Detector, Tcollect $193(2) K, 2 max $56.9 , graphite monochromated Mo-K radiation,
F(000) $1040, 10414 measured reflections, 4270 independent measured reflections, 2005 reflections with F0 2 (I).
Structure analysis and refinement. The structure was solved by direct methods (SHELXS-97) and refined by means of full-matrix least square using the program package SHELXL-97 [17, 18]. 311 parameters (non-hydrogen atoms refined anisotropically, H-atoms were included with calculated positions and refined isotropically using a riding model), R(all reflections) $0.1479, R(F0 2 F0) $0.0911, wR $0.2413, wR2 $0.2186, wR(F0 2 F0) $0.2186; GoF $0.942.
7 X-Ray Structure Determination
After addition of small amounts of ether to an acetonic solution of (1) and cooling to 20 C over several days, large prismatic crystals that contain two molecules of acetone per molecule of (1) were obtained. These crystals rapidly lose the acetone and turn into a powder at room temperature.
Crystal data. 1,4-diformyl-2,3,5,6-tetranitratopiperazine ¥ 2 (CH3)2CO, C12H18N6O16; M $502.08 g mol 1, orthorhombic,
a $ |
3 |
|
$ |
0.6631(3) nm, |
c |
$ |
2.0298(9) nm; |
V |
$ |
|||
|
1.6321(7) nm, b |
|
|
|
|
|||||||
219.7(2) nm ; |
space |
group Pca21 |
(No. 29), Z $4, |
$ |
||||||||
1519 kg m 3. Crystal dimensions 0.2 |
|
0.2 |
|
0.2 mm3, colorless |
||||||||
|
|
|
||||||||||
prism.
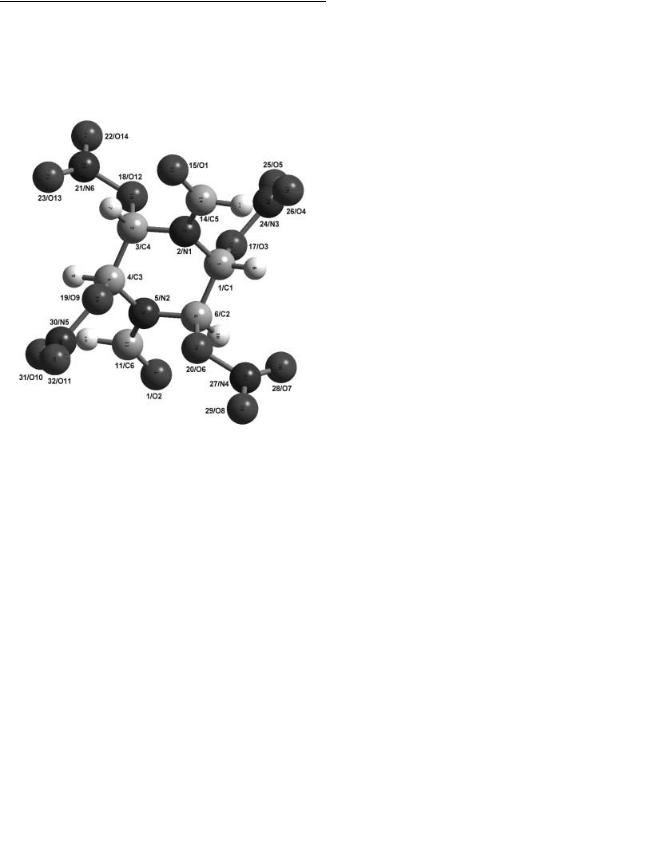
Figure 5. Molecular structure of 1,4-diformyl-2,3,5,6-tetranitra- topiperazine obtained by X-ray diffraction.
Propellants, Explosives, Pyrotechnics 28 (2003), No. 1

1,4-Diformyl-2,3,5,6-Tetranitratopiperazine |
5 |
Table 3. Comparison of the bond lengths and angles of the experimentally determined and calculated molecular structure of (1).
Bond |
Exp. (nm) |
Calc. (nm) |
Rel. deviation |
Angle |
Exp. ( ) |
Calc. ( ) |
Rel. deviation |
|
|
|
|
|
|
|
|
O1-C5 |
0.122(1) |
0.1220 |
0.7% |
O11-N5-O10 |
132.73(4) |
131.04 |
1.3% |
O2-C6 |
0.121(1) |
0.1220 |
1.6% |
O11-N5-O9 |
106.38(3) |
112.36 |
5.3% |
O3-N3 |
0.141(1) |
0.1480 |
4.1% |
O10-N5-O9 |
120.85(3) |
116.59 |
3.7% |
O3-C1 |
0.143(1) |
0.1470 |
2.5% |
N5-O9-C3 |
112.28(3) |
114.00 |
1.5% |
O4-N3 |
0.118(1) |
0.1210 |
1.7% |
O9-C3-N2 |
112.1(7) |
112.46 |
0.0% |
O5-N3 |
0.122(1) |
0.1230 |
0.8% |
O9-C3-C4 |
102.70(2) |
101.80 |
0.9% |
O6-N4 |
0.145(1) |
0.1490 |
2.6% |
N2-C3-C4 |
111.53(3) |
112.66 |
1.0% |
O6-C2 |
0.147(1) |
0.1470 |
0.1% |
N2-C6-O2 |
122.69(4) |
122.81 |
0.1% |
O7-N4 |
0.127(1) |
0.1230 |
3.1% |
N2-C2-O6 |
106.37(3) |
106.70 |
0.3% |
O8-N4 |
0.114(1) |
0.1210 |
5.4% |
N2-C2-C1 |
112.88(3) |
112.23 |
0.6% |
O9-C3 |
0.145(1) |
0.1470 |
1.3% |
O8-N4-O7 |
130.97(4) |
131.06 |
0.1% |
O9-N5 |
0.145(1) |
0.1480 |
1.9% |
O8-N4-O6 |
115.64(3) |
112.26 |
3.0% |
O10-N5 |
0.115(1) |
0.1210 |
5.5% |
O7-N4-O6 |
113.16(3) |
116.66 |
3.0% |
O11-N5 |
0.122(1) |
0.1230 |
0.8% |
C2-O6-N4 |
114.1(7) |
113.25 |
0.9% |
O12-N6 |
0.139(1) |
0.1490 |
6.5% |
O6-C2-C1 |
108.27(2) |
109.18 |
0.8% |
O12-C4 |
0.147(1) |
0.1470 |
0.1% |
C2-C1-N1 |
110.46(3) |
112.64 |
1.9% |
O13-N6 |
0.119(1) |
0.1230 |
3.1% |
C2-C1-O3 |
101.50(2) |
101.78 |
0.3% |
O14-N6 |
0.120(1) |
0.1210 |
1.1% |
N1-C1-O3 |
111.68(3) |
112.48 |
0.7% |
N1-C5 |
0.135(1) |
0.1400 |
3.9% |
O3-N3-O4 |
118.79(3) |
116.58 |
1.9% |
N1-C4 |
0.142(1) |
0.1430 |
0.6% |
O3-N3-O5 |
112.11(3) |
112.36 |
0.2% |
N1-C1 |
0.149(1) |
0.1430 |
4.3% |
O4-N3-O5 |
128.88(4) |
131.05 |
1.7% |
N2-C6 |
0.140(1) |
0.1400 |
0.6% |
N1-C5-O1 |
118.81(4) |
122.81 |
3.3% |
N2-C2 |
0.144(1) |
0.1430 |
0.8% |
N1-C4-O12 |
107.68(3) |
106.80 |
0.8% |
N2-C3 |
0.145(1) |
0.1430 |
1.5% |
N1-C4-C3 |
111.42(2) |
112.08 |
0.6% |
C1-C2 |
0.152(1) |
0.1530 |
0.9% |
C4-O12-N6 |
113.2(6) |
113.26 |
0.1% |
C3-C4 |
0.156(1) |
0.1530 |
2.0% |
C4-C3-O9 |
102.70(2) |
101.78 |
0.9% |
|
|
|
|
O12-N6-O14 |
115.74(3) |
112.29 |
3.1% |
|
|
|
|
O12-N6-O13 |
117.96(4) |
116.58 |
1.2% |
|
|
|
|
O13-N6-O14 |
126.24(4) |
131.11 |
3.7% |
Figure 6. Crystal structure of 1,4-diformyl-2,3,5,6-tetranitratopiperazine containing aceton layers.
Propellants, Explosives, Pyrotechnics 28 (2003), No. 1
6 |
K. Karaghiosoff, T. M. Klapˆtke, A. Michailovski, H. Nˆth, M. Suter and G. Holl |
The molecular structure of (1) is shown in Fig. 5, selected bond lengths and angles are given in Table 3. The X-ray structure investigation reveals the presence only of the antirotamer of (1) in the single crystal. No disorder of the formyl groups is observed. The piperazine ring adopts a chair conformation with the nitrato groups occupying the axial positions. The arrangement around the nitrogen atoms of the ring and of the nitrato groups is strictly planar. As expected the C-N distances to the carbonyl C-atoms within the amide units (0.135(1) nm and 0.141(1) nm) are significantly shorter compared to a CN single bond within the ring (0.142(1) ± 0.1491(9) nm), due to conjugation of the nitrogen lone pair with the carbonyl group. This is in accord with the hindered rotation of the formyl group around the CN bond, observed by 1H- and 13C-NMR spectroscopy in solution. The orientation of the nitrato groups of (1) in the crystal corresponds to that in the calculated structure and obviously is such as to minimize steric interactions [19, 20]. The NO3 groups at C4 and C2 are almost perpendicular to the CC bond axis of the ring with dihedral angles N-O-C-C of 86.57(3) and 90.38(3) , while the planes of the NO3 groups at C1 and C3 are almost coplanar with it (dihedral angles 158.20(3) and 157.39(3) , respectively). Bond lengths and angles within the formamido group and the nitrato groups were found to be in good accord with the data given by Bormann et al. [21] for solid dimethyl formamide and by Axenrod et al. [22] for 1,3-dinitro-5-nitrato-1,3- diazacyclohexane, respectively.
In the crystal the 1,4-diformyl-2,3,5,6-tetranitratopiperazine molecules form layers perpendicular to the c-axis with the acetone molecules filling the space between the layers (Fig. 6).
8 Conclusions
An easy preparative method giving a very high yield is described for 1,4-diformyl-2,3,5,6-tetranitratopiperazine
(1). The molecular and crystal structure of (1) is presented. A rotameric equilibrium is observed by means of 2D NMR spectroscopy. The standard heat of detonation of (1) was computed on B3PW91/6-311G(d) level of theory to be
DetH (1)calc $ 1912 kJmol 1, which corresponds to 4950 kJ kg 1. From calorimetric measurements the standard
heat of detonation was calculated to be DetH (1)exp $2059 kJ mol 1 or 5339 kJ kg 1.
9References
[1]H. Feuer and A. T. Nielsen (Eds.), Nitro Compounds, WileyVCH, Weinheim 1990.
[2]I. C. Tornieporth-Oetting and T. M. Klapˆtke, Kovalente anorganische Azide, Angew. Chem. 1995, 107, 509; Angew. Chem., Int. Ed. Engl. 1995, 34, 511.
[3]I. C. Tornieporth-Oetting and T. M. Klapˆtke, Covalent Organic Non-Metal Azides, in: I. Hargittai, T. Vidoczy (Eds.), Combustion Efficiency and Air Quality, Plenum Press, New York 1995, p. 51.
[4]T. M. Klapˆtke, Recent Developments in the Chemistry of Covalent Azides, Chem. Ber. 1997, 130, 443.
[5]D. Adam, K. Karaghiosoff, T. M. Klapˆtke, G. Holl, and M.
Kaiser, Triazidotrinitro Benzene: 1,3,5-(N3)3-2,4,6-(NO2)3C6,
Propellants, Explos. Pyrotech. 2002, 27, 7.
[6]A. C. Currie, A. H. Dinwoodie, G. Fort, and J. M. C. Thompson, Base-Catalysed Reactions of Glyoxal. Part I, J. Chem. Soc.(C), 1967, 491.
[7]T. M. Klapˆtke, C. M. Rien‰cker, Drophammer Test Investigations on Some Inorganic and Organic Azides, Propellants, Explos., Pyrotech. 2001, 26, 43.
[8]J. Akhavan, The Chemistry of Explosives, The Royal Society of Chemistry Informations Services, Herts, RSC Paperbacks
1998.
[9]www.parrinst.com
[10]M. W. Chase, Jr., NIST-JANAF Themochemical Tables, Fourth Edition, J. Phys. Chem. Ref. Data, Monograph 9,
1998, 1.
[11]J. Kˆhler and R. Meyer, Explosivstoffe, 9. Aufl., Wiley-VCH, Weinheim, 1998.
[12]Gaussian 98, Revision A.3, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr., R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle, and J. A. Pople, Gaussian, Inc., Pittsburgh PA, 1998.
[13]A. D. Becke, Density-Functional Thermochemistry III. The Role of Exact Exchange, J. Chem. Phys. 1993, 98, 5648.
[14]J. P. Perdew, Y. Wang, Phys. Rev. 1992, B 45, 13244.
[15]J. P. Perdew, K. Burke, and Y. Wang, Phys. Rev. 1996, B 54, 16533.
[16]R. Cundall, F. Palmer, and C. Wood, Vapour Pressure Measurements on Some Organic High Explosives, J. Chem. Soc., Faraday Trans. 1, 1978, 74, 1339.
[17]G. M. Sheldrick, SHELXS-97, Structure Solving Program for Crystal Structure Determination, University of Gˆttingen, Gˆttingen 1997.
[18]G. M. Sheldrick, SHELXL-97, Program for the Refinement of Crystal Structures, University of Gˆttingen, Gˆttingen 1997.
[19]A. C. Currie, A. H. Dinwoodie, G. Fort, and J. M. C. Thompson, Base-Catalysed Reactions of Glyoxal. Part II. J. Chem. Soc.(C) 1967, 497.
[20]D. Adam, G. Holl, and T. M. Klapˆtke, Nitrophenyl Azides: A Combined Experimental and Theoretical Study, Heteroat. Chem. 1999, 10, 548.
[21]H. Bormann, I. Persson, M. Sandstrom, and C. M. V. Stalhandske, The Crystal and Liquid Structures of N,N-Dime- thylthioformamide and N,N-Dimethylformamide Showing a Stronger Hydrogen Bonding Effect for C-H...S than for C- H...O, J. Chem. Soc., Perkin Trans, 2000, 2, 393.
[22]T. Axenrod, J. Sun, K. K. Das, P. R. Davy, F. Forohar et al., Synthesis and Characterization of 5-Substituted 1,3-Diazacy- clohexane Derivates, J. Org. Chem. 2000, 65, 1200.
Acknowledgements
We would like to thank Dr. C. M. Rien‰cker for performing the calorimetric measurements.
(Received August 15, 2002; Ms 2002/044)
Propellants, Explosives, Pyrotechnics 28 (2003), No. 1
