
Molecular Sieves - Science and Technology - Vol. 6 - Characterization II / 03-Characterization of the Pore Size of Molecular Sieves Using Molecular Probes
.pdf
Mol Sieves (2007) 5: 103–154 DOI 10.1007/3829_003
♥ Springer-Verlag Berlin Heidelberg 2006 Published online: 8 April 2006
Characterization of the Pore Size
of Molecular Sieves Using Molecular Probes
Yvonne Traa · Sarah Sealy · Jens Weitkamp ( )
Institute of Chemical Technology, University of Stuttgart, 70550 Stuttgart, Germany jens.weitkamp@itc.uni-stuttgart.de
1 |
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
106 |
2 |
General Aspects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
107 |
2.1 |
Dimensions of Probe Molecules and Intracrystalline Cavities . . . . . . . . |
107 |
2.2 |
Molecular Sieving . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
109 |
3 |
Adsorption of Probe Molecules with Different Sizes . . . . . . . . . . . . . |
111 |
3.1 |
Characterization of Various Zeolites in Comparison . . . . . . . . . . . . . |
111 |
3.2 |
Various Methods of Pore Size Characterization by Adsorption . . . . . . . |
116 |
3.3 |
Molecular Probes for Zeolites with Different Pore Sizes . . . . . . . . . . . |
121 |
3.3.1 |
Small-Pore Zeolites . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
121 |
3.3.2 |
Medium-Pore Zeolites . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
122 |
3.3.3 |
Large-Pore and Extra-Large-Pore Zeolites . . . . . . . . . . . . . . . . . . . |
125 |
4 |
Catalytic Test Reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
126 |
4.1 |
Shape-Selective Catalysis in Microporous Materials . . . . . . . . . . . . . |
126 |
4.2 |
Test Reactions for Monofunctional Acidic Molecular Sieves . . . . . . . . . |
129 |
4.2.1 |
Competitive Cracking of n-Hexane and 3-Methylpentane – |
|
|
The Constraint Index, CI . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
129 |
4.2.2 |
Isomerization and Disproportionation of m-Xylene . . . . . . . . . . . . . |
132 |
4.2.3 |
Other Test Reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
137 |
4.3 |
Test Reactions for Bifunctional Molecular Sieves . . . . . . . . . . . . . . . |
141 |
4.3.1Isomerization and Hydrocracking of Long-Chain n-Alkanes and Light (C7) Cycloalkanes –
|
The Refined or Modified Constraint Index, CI . . . . . . . . . . . . . . . . |
142 |
4.3.2 |
Hydrocracking of C10 Cycloalkanes such as Butylcyclohexane – |
|
|
The Spaciousness Index, SI . . . . . . . . . . . . . . . . . . . . . . . . . . . |
146 |
4.4 |
Are Monofunctional or Bifunctional Forms |
|
|
of Molecular Sieve Catalysts More Suitable? . . . . . . . . . . . . . . . . . . |
148 |
5 |
Conclusions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
149 |
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
150 |
|
Abstract This Chapter deals with the evaluation of the pore size of crystalline microporous solids with molecular probes. Only methods where the dimensions of the probe molecules and the pore width are similar are discussed. This means that the adsorption or the selectivities and/or conversions of the reaction depend, in an unambiguous manner, on the pore width. After the introduction, in Sect. 2, some general aspects are discussed that are important for the detailed understanding of the methods covered, namely dimensions of probe molecules and intracrystalline cavities as well as molecular sieving.
104 |
Y. Traa et al. |
Section 3 is devoted to adsorption, i.e., the use of molecular probes without chemical reactions. This includes mainly the characterization of various zeolites in comparison with one another and the discussion of molecular probes for zeolites with different pore sizes. Finally, in Sect. 4 shape-selective catalytic reactions are reviewed which have been employed for characterizing the width of micropores. As an introduction, the basics of shape-selective catalysis in microporous materials are discussed. The main test reactions dealt with are, for monofunctional acidic molecular sieves, the competitive cracking of n-hexane and 3-methylpentane (Constraint Index) as well as the isomerization and disproportionation of m-xylene. For bifunctional molecular sieves, the isomerization and hydrocracking of long-chain n-alkanes (Refined or Modified Constraint Index) as well as the hydrocracking of C10 cycloalkanes such as butylcylohexane (Spaciousness Index) are reviewed.
Abbreviations
aadsorbed in the pores
a/e |
adsorbed very slowly and in small amounts in the pores |
AFI |
three-letter code of the International Zeolite Association for zeolite SSZ-24 |
AFR |
three-letter code of the International Zeolite Association for zeolite SAPO-40 |
BEA |
three-letter code of the International Zeolite Association for zeolite Beta |
BET |
Brunauer Emmett Teller |
CFI |
three-letter code of the International Zeolite Association for zeolite CIT-5 |
CHA |
three-letter code of the International Zeolite Association for zeolite chabazite |
CI |
Constraint Index |
CI |
Refined or Modified Constraint Index |
CIT |
California Institute of Technology |
CON |
three-letter code of the International Zeolite Association for zeolite CIT-1 |
C8S.I. |
C8 Selectivity Index |
CVD |
chemical vapor deposition |
DDR |
three-letter code of the International Zeolite Association for zeolites decado- |
|
decasil 3R, Sigma-1 |
DFT |
density functional theory |
2,2-DM-Bu 2,2-dimethylbutane |
|
DON |
three-letter code of the International Zeolite Association for zeolite UTD-1 |
eexcluded from the pores
EMC |
Elf Mulhouse Chimie |
EMT |
three-letter code of the International Zeolite Association for zeolite EMC-2 |
ERI |
three-letter code of the International Zeolite Association for zeolite erionite |
EU |
Edinburgh University |
EUO |
three-letter code of the International Zeolite Association for zeolites ZSM-50, |
|
EU-1 |
FAU |
three-letter code of the International Zeolite Association for zeolites X and Y |
FER |
three-letter code of the International Zeolite Association for zeolites ZSM-35, |
|
ferrierite |
FT-IR |
Fourier transform infrared |
n-Hx |
n-hexane |
HPLC |
high-pressure liquid chromatography |
IFR |
three-letter code of the International Zeolite Association for zeolite ITQ-4 |
IR |
infrared |
ITQ |
Instituto de Tecnologia Quimica Valencia |
Characterization of the Pore Size of Molecular Sieves |
105 |
|
IZA |
International Zeolite Association |
|
kfirst-order rate constants
KFI |
three-letter code of the International Zeolite Association for zeolite ZK-5 |
LHSV |
liquid hourly space velocity |
LTA |
three-letter code of the International Zeolite Association for zeolite A |
LTL |
three-letter code of the International Zeolite Association for zeolite L |
mmass
MAZ |
three-letter code of the International Zeolite Association for zeolite Omega |
MCM |
Mobil composition of matter |
M-CPn |
methylcyclopentane |
MEA |
multiple-equilibrium analysis |
MEL |
three-letter code of the International Zeolite Association for zeolite ZSM-11 |
MFI |
three-letter code of the International Zeolite Association for zeolite ZSM-5 |
MIN-1 |
minimum dimension through a molecule |
MIN-2 |
second minimum dimension through the same molecule perpendicular to |
|
MIN-1 |
3-M-Pn |
3-methylpentane |
2-M-Pr |
2-methylpropane |
MOR |
three-letter code of the International Zeolite Association for zeolite mordenite |
MTG |
methanol to gasoline |
MTH |
methanol to hydrocarbons |
MTO |
methanol to olefins |
MTT |
three-letter code of the International Zeolite Association for zeolite ZSM-23 |
MTW |
three-letter code of the International Zeolite Association for zeolite ZSM-12 |
MWW |
three-letter code of the International Zeolite Association for zeolites MCM- |
|
22, SSZ-25, ITQ-1 |
nmolar amount
n˙ |
molar flow |
NES |
three-letter code of the International Zeolite Association for zeolite NU-87 |
NMR |
nuclear magnetic resonance |
NU |
new (ICI) |
pi |
partial pressure at the adsorber outlet |
pi,0 |
partial pressure at the adsorber inlet |
rdistance
Rratio of rate constants observed experimentally for the formation of o- and
|
p-xylene from m-xylene under conditions of diffusional limitations |
RCN |
index defined as the ratio of the adsorption capacity of cyclohexane to that |
|
of n-hexane |
Sselectivity
SAPO |
silicoaluminophosphate |
SI |
Spaciousness Index |
SR |
Selectivity Ratio |
SSI |
Shape Selectivity Index |
SSZ |
Standard Oil synthetic zeolite |
t0.3 |
time for o-xylene sorption of 30% of the p-xylene capacity |
Ttemperature
TIPB |
1,3,5-triisopropylbenzene |
TON |
three-letter code of the International Zeolite Association for zeolite ZSM-22 |
TOS |
time on stream |
TPD |
temperature-programmed desorption |
106 |
Y. Traa et al. |
type A |
the classification of β-scissions of alkyl carbenium ions has been introduced |
β-scission |
in [120]. The salient feature of type A β-scissions is that they start from |
|
a tertiary and lead again to a tertiary carbenium ion |
type B |
The classification of β-scissions of alkyl carbenium ions has been introduced |
β-scission |
in [120]. The salient feature of type B β-scissions is that they start from |
|
a tertiary and lead to a secondary carbenium ion or vice versa |
UTD |
University of Texas at Dallas |
UV/Vis |
ultraviolet/visible |
˙ |
volumetric flow rate |
V |
|
VFI |
three-letter code of the International Zeolite Association for zeolite VPI-5 |
VPI |
Virginia Polytechnic Institute |
VROA |
relative o-xylene adsorption velocity |
Xconversion
Yyield
ZK |
zeolite Kerr |
ZSM |
Zeolite Socony Mobil |
φzeolite Socony Mobil potential
σ“kinetic diameter”, which appears as a parameter in the Lennard-Jones potential
1 Introduction
With molecular probes, various properties of crystalline microporous solids can be explored. One example is the determination of surface acidity/basicity by adsorption and desorption of basic/acidic probe molecules (e.g., ammonia, pyridine, carbon dioxide, chloroform or deuterochloroform) (cf. this book series, vol 7, Chap. 1) and observing the sorption processes by IR spectroscopy (vibrational spectroscopy) (cf. this book series, vol 4, Chap. 1), NMR spectroscopy (cf. this book series, vol 4, Chap. 2), mass spectrometry or gas chromatography. Another possibility is the evaluation of surface hydrophobicity/hydrophilicity by sorption of mixtures of non-polar and polar substances. The assessment of micropore volume and pore size can be accomplished by adsorption of xenon monitored by 129Xe NMR (cf. this book series, vol 5, Chap. 4) spectroscopy, by adsorption of nitrogen and other small molecules usually followed gravimetrically or volumetrically (cf. this book series, vol 6, Chap. 4) or by determining the heats of adsorption by means of microcalorimetry (cf. this book series, vol 6, Chap. 5).
This chapter deals with the evaluation of the pore size of crystalline microporous solids with molecular probes, but only those methods that are based on size effects will be discussed, i.e., where the dimensions of the probe molecules (or of the transition states/product molecules formed from them) and the pore width are similar. These methods include adsorption of molecules of different sizes large enough to “feel” the presence of the micropores and, therefore, allowing an assessment of the pore width [1], and test
Characterization of the Pore Size of Molecular Sieves |
107 |
reactions in which the selectivities and/or conversions depend, in an unambiguous manner, on the pore width, i.e., shape-selective reactions. In the early days of zeolite science, these two techniques were most popular tools for collecting information on the approximate crystallographic pore size of zeolites with unknown structures. With the advent of more sophisticated and highly efficient crystallographic methods, a rapid determination even of complex new structures became feasible. Hence, the initial incentive for the application of methods using molecular probes has shifted [2]: Nowadays, these techniques are primarily used as quick tests for probing the effective pore width under catalytically relevant conditions and/or of molecular sieves manipulated and modified with post-synthesis methods such as chemical vapor deposition (CVD), deliberate or unwanted coking, isomorphous substitution of framework atoms and the like.
In Sect. 2, some general aspects will be discussed that are important for the detailed understanding of the methods covered. Section 3 will be devoted to adsorption, i.e., the use of molecular probes without chemical reactions. Finally, in Sect. 4 shape-selective catalytic reactions, which have been employed for characterizing the width of micropores, will be reviewed.
2
General Aspects
2.1
Dimensions of Probe Molecules and Intracrystalline Cavities
For the discussion of size effects, it is vital that the dimensions be defined in an appropriate way. This problem was tackled in an excellent paper by Cook and Conner [3]. These authors stress that the hard-sphere picture underlies the thinking about adsorption, i.e., adsorbate molecules and the adsorbent are generally both considered to be rigid structures composed of hard-sphere atoms or ions. However, in reality the molecules and the host matrix are in continuous vibration and often quite flexible. Therefore, if results of adsorption experiments are to be explained using the hard-sphere picture, the dimensions of the probe molecules and of the intracrystalline cavities should be brought in line with this simple picture. In other words, the model employed for interpreting the adsorption process should be consistent with the type of dimensions used for the description of the probe molecules and the intracrystalline cavities. By contrast, the “kinetic diameter” σ (from the Lennard-Jones potential, cf. Fig. 1) of the adsorbate and the pore size based on the ionic oxygen radius of 0.135 nm are generally used, and these dimensions do not satisfy this criterion of consistency [3]. In many instances, molecules do diffuse into pores that are considered to possess a width lower than the molecular diameter. An example is cyclohexane (σ = 0.60 nm, [4])
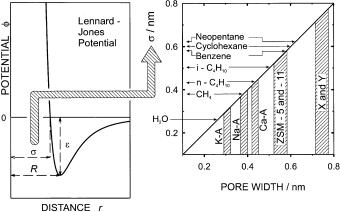
108 |
Y. Traa et al. |
Fig. 1 Dimensions of probe molecules and intracrystalline cavities (σ is the “kinetic diameter” that appears as a parameter in the Lennard-Jones potential)
in zeolite ZSM-5 (0.53 × 0.56 and 0.51 × 0.55 nm, [5], cf. Fig. 1) [6]. For this reason, Cook and Conner [3] proposed a re-definition of framework atom sizes, based on the average physical extension of electron density distributions in zeolite frameworks. With this modification, the maximum dimension of the ZSM-5 pores would be 0.63 nm, now permitting cyclohexane to enter its pores.
Another fact that should be taken into account is that, for cylindrical pores, the consideration of just one molecular dimension is usually insufficient. Hardly any molecule has spherical symmetry. In this context, Webster et al. [7] advanced the concept of effective minimum dimensions of molecules. MIN-1, the minimum dimension through a molecule, and MIN-2, the second minimum dimension through the same molecule perpendicular to MIN-1, determine whether or not this molecule can enter the pores of a given material. These dimensions can be calculated with molecular orbital theory and allow a more sophisticated description of the molecular behavior inside the pores.
Furthermore, one should consider the flexibility of molecules and how this flexibility can affect the diffusion and adsorption inside the pores. Choudhary and Akolekar [8] proposed the shuttlecock-shuttlebox model to account for the fact that larger molecules than expected do diffuse into pores of a given width. Their model envisages the compression of alkyl groups of branched molecules similar to the compression of feathers of a shuttlecock in a shuttlebox.
In addition, one should keep in mind that the pore dimensions given in the “Atlas of Zeolite Framework Types” [5] are coarse data and subject to changes due to both the experimental conditions and the precise form of the porous material. For example, the effective pore dimension varies with temperature. The best way for probing this are catalytic tests performed at
Characterization of the Pore Size of Molecular Sieves |
109 |
different temperatures [9]. This will be discussed in more detail in Sect. 4. Another parameter that can affect the pore size is the aluminum content: Framework aluminum tends to reduce the pore volume and to broaden the pore size distribution. Steam treatment has been reported to reduce the apparent pore size [10]. Furthermore, the shape of the pore aperture can change during adsorption [4], and zeolites can undergo structural rearrangement, which might alter the pore size [11]. Wu and Ma [12] showed that the adsorption capacity for various hydrocarbons on zeolite ZSM-5 decreases as the radius of the cation increases. Thus, certain parts of the channel system can be blocked by cations, and the pore aperture can be contracted. In the extreme case, molecules are excluded from the pore system. For example, uncalcined offretite with large organic cations in its channels adsorbs neither cyclohexane nor n-hexane, whereas the calcined zeolite does [13]. In the “Atlas of Zeolite Framework Types” [5], the pore size of the species examined first is given. In zeolite minerals, different cations are often present, which reduce the pore size, compared to the synthetic zeolites and the pure silica analogues, which undergo no such reduction. Thus, the reader should pay attention to the exact form of the species the pore size of which was determined. Another possible reason for pore blockage is amorphous material in the intracrystalline cavities [14]. Finally, preadsorption of polar molecules such as water or ammonia often affects the subsequent adsorption of other molecules by clustering around the cations, reducing the apparent pore size and adsorption capacity and eventually blocking the pores [15]. Therefore, particular attention should always be paid to the hydration state of the zeolite.
In conclusion, one should be very careful when predicting whether or not a given molecule has access to the pores of a given material merely from tabulated sizes of molecules and cavities. Only if the dimensions of the probe molecules and the dimensions of the intracrystalline cavities are chosen in such a way that they are consistent with each other and the adsorption model, meaningful predictions are possible [9].
2.2
Molecular Sieving
Molecular sieving is the selective adsorption of molecules into the intracrystalline void system of a molecular sieve and the exclusion of others due to their dimensions being above the critical size. One example is illustrated in Fig. 2: A gaseous mixture of n-pentane and 2-methylbutane was continuously passed over a fixed bed of calcium-exchanged zeolite A (or zeolite “5A”) with
an effective pore diameter of ca. 0.5 nm (5 ˚). This is large enough to al-
A
low for the diffusion of the n-alkane molecules through the 8-membered ring windows of zeolite A, but too small for the uptake of the branched alkane. Hence, 2-methylbutane breaks through at the adsorber outlet directly after the onset of the experiment, while the smaller n-pentane is completely ad-
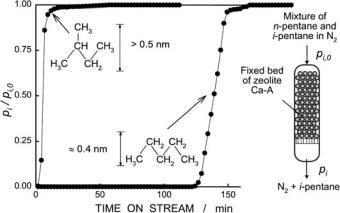
110 |
Y. Traa et al. |
Fig. 2 Breakthrough curves for the adsorption of an n-pentane/2-methylbutane mixture over zeolite Ca-A in a fixed-bed flow-type adsorber
sorbed by the zeolite for about 2 h, whereupon its adsorption capacity is exhausted.
The example displayed in Fig. 2 represents the extreme case in molecular sieving, namely pore size exclusion, i.e., one molecular species is so bulky that it is completely prevented from entering the intracrystalline cavities. In many instances, however, molecules do enter the pores, but their diffusion inside the pores is very slow. In such cases, very different results for the adsorption capacity will be obtained depending on whether or not the system was given enough time to reach adsorption equilibrium. Furthermore, for the purpose of pore size characterization, one must keep in mind that adsorptive separation on zeolites can be accomplished not only by molecular sieving, but also by selective cation-adsorbate interaction, by selective sorption due to hydrophilic or hydrophobic surface properties or by selective sorption due to acidic/basic surface properties. The discussion in this chapter will essentially be restricted to molecular sieving, however, a clearcut distinction between the different mechanisms of adsorptive separation is sometimes difficult.
Obviously, if adsorption at the external surface of a molecular sieve occurs to a significant extent, this will obscure the desired information on the pore size. Masuda and Hashimoto [16] demonstrated that up to 50% of the total amount adsorbed can be located on the external surface, if the zeolite crystals are very small. One possibility to eliminate the undesired adsorption of the probe molecule(s) on the external surface is to add another component (e.g., as a solvent in liquid-phase adsorption), the molecules of which are so bulky that they are completely hindered from entering the pores. These bulky molecules will often cover the external surface exclusively, especially if their concentration in the bulk fluid phase is high.
Characterization of the Pore Size of Molecular Sieves |
111 |
Another complication arises when the size of the probe molecules closely matches the size of the pore. Under these conditions, branched isomers can be more strongly adsorbed than linear ones. For example, Santilli et al. [17] reported that for zeolites of pore sizes between 0.6 and 0.7 nm, there was an increased preference for branched versus linear hexane isomers, giving rise to inverse shape selectivity. This phenomenon should be borne in mind when adsorption over a range of pore sizes is being studied. Santilli et al. [17] argued that attractive forces between the zeolite walls and hexane isomers stabilize the branched isomers relative to n-hexane. Schenk et al. [18], on the other hand, were of the opinion that n-hexane is excluded from the pores because its effective size is greater, i.e., adsorption of shorter, more compact isohexanes is preferred to that of the longer n-hexane. This was demonstrated using Grand Canonical Monte Carlo simulations and points to the value of using molecular simulations in understanding adsorbate-absorbent interactions, especially in relation to shape-selective effects.
Wu et al. [19] pointed out a few more pitfalls: They found that the amount of cyclohexane sorbed in large crystals of zeolite ZSM-5 was much lower after 2 h of equilibration than that sorbed in smaller crystals of the same zeolite. This observation was explained by increasing time required to reach adsorption equilibrium with increasing crystallite size [19]. Another fact that could hinder the adsorption are imperfections of the large crystals, which are invisible with conventional characterization techniques. Therefore, when interpreting adsorption results, one should always take into account the crystallite size of the samples. Another factor that can severely affect the results are impurities in the adsorptives [19]. Even at low concentrations, impurities in an adsorptive can lead to erroneous results due to selective adsorption of the impurity, especially if its molecular dimensions are small.
3
Adsorption of Probe Molecules with Different Sizes
Probing the pore width of microporous materials by adsorptives of different molecular size has been a popular method since the beginning of zeolite science. Consequently, a vast amount of results are scattered in the scientific and patent literature. No comprehensive discussion of these results is aimed at in the present review. Rather, a few selected and instructive examples will be presented.
3.1
Characterization of Various Zeolites in Comparison
Adsorption of an appropriate set of components with different molecular dimensions is a widely accepted procedure for characterizing the pore sizes of

112 Y. Traa et al.
zeolites. A very thorough study on several 8-, 10and 12-membered-ring zeolites was made by Wu et al. [19], which can be looked upon as a basis for a large part of the later work. During static adsorption at room temperature, substantial amounts of n-hexane were sorbed by all zeolite samples, since its critical dimensions are smaller than or equal to their crystallographic pore openings. The largest adsorptive, mesitylene (1,3,5-trimethylbenzene), was sorbed only in the pores of the 12-membered-ring zeolites. The authors concluded that the ability to sorb benzene and cyclohexane classifies zeolite ZSM-23 (MTT) (the structural code of the International Zeolite Association is given in parentheses [5]) and ZSM-48 as medium-pore zeolites. However, having only one-dimensional channels, zeolites ZSM-23 and ZSM-48 adsorbed less benzene or cyclohexane than zeolites ZSM-5 (MFI) and ZSM11 (MEL), which have intersecting 10-membered-ring channels. The lower adsorption capacity of the 12-membered-ring zeolite ZSM-12 (MTW) as compared to zeolite Y (FAU) with its very spacious pore system was proposed to be a consequence of its denser structure with one-dimensional channels (like the large-pore zeolite mordenite (MOR); the 8-membered-ring pore channels of mordenite do not adsorb the hydrocarbons used). The lesser amount of mesitylene sorbed by ZSM-12 as compared to mordenite was said to be consistent with its smaller crystallographic pore opening, impeding the diffusion of the large mesitylene molecules into its intracrystalline voids [19].
Wu et al. [19] also applied a dynamic adsorption method using a thermogravimetric analyzer. These experiments were carried out at 373 K; equilibrium was judged to have been achieved when the weight gain was less than 17 ng/s. ZSM-23 and ZSM-48 exhibited a comparable capacity ratio of 3-methylpentane to n-hexane as ZSM-5 and ZSM-11, but a considerably lower ratio of uptake rate for these two hydrocarbons, reflecting again the unidimensionality of the channels in ZSM-23 and ZSM-48. However, the capacity of cyclohexane sorbed by ZSM-48 was larger and the rate of uptake faster than on ZSM-23, consistent with the greater eccentricity of the pore openings and the channels of ZSM-23.
In a further evaluation of their experimental data, Wu et al. [19] determined effective pore sizes of the zeolites by using the smaller cycloalkanes as a measure of the minor axis and the larger aromatics as a measure of the major axis of the effective pore opening. Just one example is given here for illustration: Since the uptake rate of cyclohexane (about 0.5 × 0.6 nm size) in ZSM-23 was very low, the minor axis of the ZSM-23 channels was concluded to be about 0.45 nm, approaching that of the smallest dimension of cyclohexane. Its ability to sorb p-xylene (about 0.4 × 0.6 nm size) and o-xylene less readily (about 0.4 × 0.7 nm size) indicates that its major axis appears to be only 0.65 nm. Table 1 gives an overview of which molecules can enter the pores of important zeolites. When using this table, the reader should always be aware that the adsorption behavior is dependent on the experimental con-
