
Molecular Sieves - Science and Technology - Vol. 6 - Characterization II / 02-Thermal Analysis of Zeolites
.pdf
Mol Sieves (2007) 5: 67–101 DOI 10.1007/3829_002
♥ Springer-Verlag Berlin Heidelberg 2005 Published online: 20 December 2005
Thermal Analysis of Zeolites
Gabriella Pál-Borbély
Institute of Chemistry, Chemical Research Center, Hungarian Academy of Sciences, Pusztaszeri ut´ 59–67, 1025 Budapest, Hungary
borbely@chemres.hu
1 |
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
67 |
2 |
Characterization of Zeolites by Thermal Analysis . . . . . . . . . . . . . . |
69 |
3 |
Chemical Modification of Zeolites Traced by Thermal Analysis . . . . . . |
75 |
4 |
Thermal Removal and Decomposition of Templates Occluded in Zeolites . |
76 |
5 |
Characterization of Isomorphously Substituted Zeolites . . . . . . . . . . |
88 |
6Characterization of Zeolites by Thermal Analysis
of Adsorbed Alkylamines and Alcohols . . . . . . . . . . . . . . . . . . . . |
92 |
7Thermal Analysis of Alkylamines Adsorbed on Metal Ions
|
Located in Cationic Positions of the Zeolite Framework . . . . . . . . . . . |
95 |
8 |
Conclusions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
98 |
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
98 |
|
1 Introduction
In general, thermal analysis can provide important information on the temperature-dependent properties (heat capacity, thermal stability) of materials and on thermally induced processes (phase transition, decomposition, etc.). Thermal analysis is advantageous in that it quickly gives a general view of the thermal behavior of a material under various conditions and requires a small amount of sample.
The fundamentals of the four most frequently applied methods—thermo- gravimetry, differential thermal analysis, differential scanning calorimetry, and dilatometry—are described and illustrated with examples of applications in the field of preparative chemistry in [1]. As to zeolite investigations by microcalorimetry, a comprehensive review with 69 references has been published recently [2].
In the case of thermogravimetry (TG), the change in the weight of a sample is measured as a function of temperature (or of time in the case of isothermal
68 |
G. Pál-Borbély |
measurements). Measurements in inert or reactive gases, even in vacuum, are possible over a wide temperature range with a constant heating rate or using different non-linear temperature programs. The derivative thermogravimetry (DTG) indicates the rate of the mass loss ( dm/ dt). Differential thermal analysis (DTA) is based on the measurement of the temperature difference, ∆T, between the sample and an inert material (reference) during a temperature program. The temperature can be measured either near or directly inside the sample and reference. At thermal equilibrium the oven, the sample, and the inert material have the same temperature. When a thermal event occurs in the sample during the heating process, the stationary state will be disturbed. The temperature of the sample can be higher (exothermic reaction) or lower (endothermic reaction) than the temperature of the inert material. ∆T is plotted against temperature or time. Differential scanning calorimetry (DSC) monitors the change in enthalpy ( dH/ dt). In the technique of dilatometry changes in the length of the sample are examined during the heating process.
Thermoanalytical methods were often used simultaneously and combined with complementary methods. In this field the main attention was paid to the decomposition reactions of solids accompanied by gas evolution. Many experimental techniques combining the thermogravimetry with evolved gas analysis [gas chromatography (GC), mass spectrometry (MS)] were developed. These methods led to conclusions drawn about the reactions due to the observed changes in weight. The widespread simultaneous application of TG and DTA enabled a specific change in mass to be attributed to a thermal event. Many papers on characterization of zeolites and clays were published, in which emphasis was laid on the advantages of combining simultaneous thermoanalytical methods (TG-DTA) with other complementary techniques (e.g. MS, GC, XRD, IR). Fundamentals and fields of application of temperatureresolved X-ray and neutron diffraction methods were presented by Epple [3].
Gimzewski [4] proposed the application of high-pressure thermogravimetry for the measurement of gas sorption on solids and for the study of solids (for instance catalysts) under industrially realistic conditions with respect to temperature and pressure.
Langier-Kuzniarowa [5] has recently reviewed the application of thermal methods for the study of clay minerals and rocks. From this comprehensive work it turned out that a large number of papers dealt with sorption properties of minerals and with organo-clay complexes. Pillared and intercalated clays were frequently subjected to thermal examinations. TG and DTA were used to investigate kinetics and thermodynamic parameters of dehydration and dehydroxylation of clay minerals.
Thermal analysis has also been shown to be a valuable technique for the characterization of zeolites. Combined TG and DTA provided information on the dehydration behavior and stability of zeolites. The decomposition of organic molecules (ions) incorporated in the zeolitic channel system during
Thermal Analysis of Zeolites |
69 |
the synthesis has been extensively studied since nitrogen-containing organic compounds are being used in the synthesis of high-silica zeolites. The temperature range in which the organics are removed from the cages and channels of zeolites is of great significance for the application of these materials in industrial processes (catalysis, adsorption).
In a review of predominantly the author’s own papers, Gabelica et al. [6] presented very illustrative examples of applications of thermoanalytical techniques for the investigation of synthesis and properties of pentasil zeolites. Weight losses (TG) and heat effects (DTA) due to the oxidative decomposition of tetrapropylammonium ions filling the channel structure of the zeolite were related to the amount of ZSM-5 formed during the crystallization. Thermal analysis was shown to be a very accurate technique for discriminating between loosely bound TPA ions and those interacting strongly with a zeolite-type framework. Thus, very small X-ray amorphous particles of ZSM-5 zeolite could be detected in the early stages of the crystallization process. Thermal analysis could be used to monitor slight changes in the free pore volume of some modified zeolites by measuring the adsorption and desorption of small hydrocarbon molecules. Furthermore, the technique made it possible to monitor the formation and removal of coke deposits.
The present review covers literature of the last 15–20 years dealing with the application of thermoanalytical methods in the field of zeolite chemistry. The aim is to show that thermal analysis is an efficient tool for physical-chemical characterization of zeolites.
2
Characterization of Zeolites by Thermal Analysis
Thermogravimetry was applied to measure the amounts of water adsorbed on sodium, calcium, and potassium forms of faujasites with different Si/Al ratios [7]. The number of water molecules per u.c. was found to increase with decreasing Si/Al ratio.
Differential scanning calorimetry was used to study the thermal effects during adsorption and desorption of water on zeolite NaA in the form of powder or thin layers [8]. The amounts of water adsorbed after preceding activation and at different temperatures were thermogravimetrically measured, and the appropriate adsorption heats were determined by DSC. On the basis of the relation between both characteristics, the mass of the zeolite in thin layers deposited on metals was obtained from the DSC results.
The dehydration of a new microporous cesium silicotitanate (SNL-B) molecular sieve and subsequent readsorption of water on it were measured by TG [9]. An observed difference between the amount of desorbed and readsorbed water was attributed to the irreversible dehydration of a second phase present as an impurity.
70 |
G. Pál-Borbély |
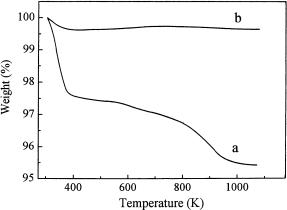
Defect sites created during the dealumination of Na-Y with SiCl4 vapor and subsequent acid washing were characterized by IR, 29Si (CP) MAS NMR spectroscopy, and TG [10]. A rapid weight loss up to 373 K revealed by the TG curve of the dealuminated Y zeolite (see Fig. 1) was undoubtedly due to the release of physisorbed water, while the slower weight loss between 573 and 973 K could be attributed to the loss of water formed as a result of the healing of defects through condensation of silanol groups. Steaming resulted in a very low sorption capacity of dealuminated Y for water and in the healing of defects. Accordingly, the weight loss of the sample due to dehydration and dehydroxylation of silanol groups was found to be very small. The zeolite became hydrophobic.
An attempt was made to quantify the hydrophobicity (H) of zeolites by thermogravimetric analysis [11]. The value of H was related to the amount of the adsorbed water determined by TG and defined as the ratio of the weight loss at 423 K to that at 673 K. Weitkamp et al. [12] pointed to the inadequacy of the H parameter, and they proposed for the characterization of the hydrophobic properties of modified zeolites the use of the Hydrophobicity Index (HI) which was based on competitive adsorption of toluene and water
from the gas phase. HI was defined as Xtoluene/XH2O, where X is the loading i.e., the mass of adsorbed compound per mass of dry adsorbent.
High-siliceous zeolites of the ZSM-5 type show hydrophobic/organophilic properties. TG/DTG/DTA was used for quick characterization of the affinity of zeolites to organics [13]. A new concept concerning the affinity, AT, of the perfect micropore surface in silicalite-1 to an adsorbed organic compound was developed. This quantity was defined as AT = Td – Tb, where Td is the temperature of the weight loss peak in the DTG curve and Tb the boiling point of the organic compound. AT values were used to compare
Fig. 1 TG curves of zeolite Y a dealuminated with SiCl4 vapor at 673 K followed by acid washing, b after subsequent steaming at 993 K (reproduced from [10])
Thermal Analysis of Zeolites |
71 |
the affinity order of 27 organic compounds. In later papers, the interactions between the framework of high-siliceous hydrophobic FAU [14] and ferrierite type zeolite [15] and 25 adsorbed organic compounds were studied in a similar way by TG/DTG/DTA. Hydrocarbons, alcohols, organic acids, alkylamines, and esters were applied as adsorbates. The AT values of organics adsorbed on siliceous FAU and silicalite-1 were found to be similar in their graduation. These zeolites, built up exclusively from SiO4 tetrahedra, had a stronger affinity to saturated hydrocarbons than for unsaturated ones and exhibited hydrophobic behavior towards alkylalcohols and organic acids. Special sorbate/framework and sorbate/sorbate interactions were found for amines. It was pointed out that the AT values might be useful to estimate the feasibility and effectiveness of separation processes in practice.
Usually, an exothermic peak in the high-temperature interval of the DTA curve was found to hint at a loss of crystallinity [16–19]. The temperature at which the exothermic peak associated with lattice collapse appeared in the DTA curve was used to characterize the thermal stability of faujasites as a function of the Si/Al ratio, cation type (La3+, Ca2+ , Na+) and degree of ion exchange [20].
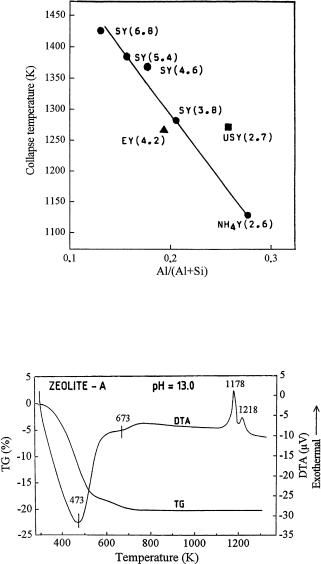
The thermal stability of zeolite Y dealuminated hydrothermally (USY) and by Al-extraction with (NH4)2SiF6 (SY) or EDTA (EY) was also studied by DTA [21]. The onset temperature of the exothermic DTA peak was considered as the temperature of crystal collapse. Figure 2 shows that the thermal stability of SY samples increased almost linearly with the decrease of their Al/(Al + Si) ratios. The framework of NH4 – Y (Si/Al = 2.7) collapsed at about 1090 K while SY (Si/Al = 6.8) was stable up to 1387 K. Dealumination by H4EDTA solution resulted in a smaller increase in the thermal stability of the zeolite. From the point of view of framework stabilization, the substitution of silicon for aluminum by (NH4)2SiF6 treatment proved to be more effective than the extraction of aluminum with H4EDTA.
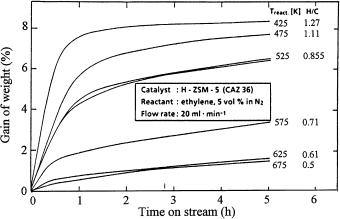
The exothermic lattice breakdown of zeolite Na-A, which leads to typical doublet peaks in the DTA curve at around 1200 K, was employed to check the success of crystallization of zeolite Na-A from synthesis mixtures at various pH values [22]. The TG and DTA curves of a fully crystallized zeolite Na-A are presented in Fig. 3.
Also, phase transition and destruction of the structure of natural heulandites and clinoptilolites were investigated by differential thermal analysis [23]. A relationship was found between the chemical composition (cation form) and the thermal behavior of clinoptilolites. Differential scanning calorimetric measurements of ethanolamineand ethylenediamine-silica sodalite pointed to temperature-induced phase transitions in the temperature range of 100–300 K [24].
Dilatometry and derivative dilatometry combined with DTA are good methods for measuring and continuous recording of lattice deformations,
72 |
G. Pál-Borbély |
Fig. 2 Crystal collapse temperatures determined by DTA as a function of Al/(Al + Si) ratio of parent Y zeolite (NH4-Y) and varieties dealuminated hydrothermally (USY) and by treatment with (NH4)2[SiF6] (SY) and H4 EDTA (EY). The figures in parentheses indicate the Si/Al ratio (reproduced from [21])
Fig. 3 TG and DTA curves of zeolite Na-A synthesized at pH = 13 (reproduced from [22])
metaphase formations, and phase transitions during the thermal dehydration of zeolites, provided these transitions are accompanied by a pronounced expansion or contraction. Ullrich et al. [25] demonstrated that the dehydration of minerals of the natrolite group is accompanied by an expansive process, while that of stilbite and heulandite proceeds under contraction, depending on the water bonding in the zeolite lattice.
Thermal Analysis of Zeolites |
73 |
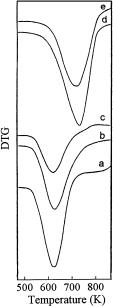
Catalyst deactivation is most frequently due to the formation of carbonaceous residues in acid-catalyzed hydrocarbon reactions. Techniques and apparatus suitable for the investigation of coke formation and their application illustrated with examples were reviewed by Karge [26]. It was shown that thermogravimetric measurements and TGA combined with GS and MS could provide useful data concerning the amount of coke deposits and their H/C ratios. In the procedures presented in [26], the products of the coke combustion (H2O, CO2 ) were trapped and analyzed by GS and the weight loss was measured simultaneously. The TG technique was not only used to determine the H/C ratio of coke but also to trace the coke formation passing the gaseous reactant over the catalyst in situ in the microbalance. In this case, the amount of carbonaceous deposits was monitored via the weight gain of the catalyst. As an example, Fig. 4 shows the weight gain vs. time on stream due to in situ coke formation during ethylene conversion on a ZSM-5-based catalyst at reaction temperatures increasing from 425 to 675 K. Also, the H/C ratios after 6 h on stream are indicated.
Significant influence of reactant dilution and chemical nature of the carrier gases on composition and concentration of coke residues deposited on zeolite Beta during the catalytic disproportionation of cumene was found to be reflected by the thermograms and 13C NMR spectra of the thus-deactivated catalysts [27]. The nature of coke (so-called soft or hard coke) and the removal of these deposits from the catalyst were found to be strongly influenced by the reaction conditions (e.g. temperature, space velocity) during the aromatization of propane on MFI type H-gallosilicate [28]. The results of TA supported that, in contrast to the high-temperature residues, the coke formed at low temperature was volatile. A combined technique consisting of TG and
Fig. 4 Weight gain due to coke formation during ethylene conversion over H-ZSM-5 at different reaction temperatures (reproduced from [26])
74 |
G. Pál-Borbély |
continuous detection of evolved products by IR was applied to study the removal of carbonaceous deposits previously formed during the conversion of methanol to hydrocarbons on zeolite H-ZSM-5 [29]. In different gas streams (e.g. helium, hydrogen, and propane) the effect of thermal treatments on the removal of coke and the changes during these treatments in the nature of coke were investigated.
The formation of carbonaceous deposits during the alkylation of isobutane with 1-butene over La,M-Y (M = alkali or alkaline earth element) was studied by TA in [30]. Two types of carbonaceous material could be distinguished:
(i) deposits at the pore mouth removable by desorption at temperatures below 573 K and (ii) olefinic oligomers located in the internal pore space resulting in a decrease of the BET surface and in a deactivation of the catalyst.
In another study [31] coked Mo/H-ZSM-5 zeolite previously used as a catalyst for the dehydro-aromatization of methane was subjected to different temperature-programmed thermal treatments in reducing and oxidizing atmospheres. Then TG was applied to determine the amount of coke after the respective treatments and to gain in this way information on the burning-off process.
Until the mid-nineties conventional dynamic balances played a decisive role as a central measuring instrument in thermoanalytical methods. However, the need for direct mass determination in weightless conditions (e.g. in space vehicles) led to the development of a new type of so-called TEOM (tapered element oscillating microbalance) systems. The mass of the sample located at the tip of an oscillating quartz element is measured by the vibrational frequency of the system. This new instrumental technique became commercialized in recent years and instantly utilized in thermoanalyzers. In conventional microbalance reactors a large part of the feed bypasses the catalyst sample which makes it difficult to confirm gradientless differential operation. In contrast, in the TEOM reactor all the fed reactant (or adsorbate) passes the catalyst (adsorbent) bed even at high flow rates of the carrier gas similarly to the flow conditions in a catalytic fixed-bed reactor (or adsorption column). Thus, external mass and heat transfer limitations play a minor role in model equations for the determination of kinetic data from mass change patterns operated in the non-linear range. Chen et al. [32] highlighted the advantages of this new measuring principle in a thermoanalytical study of the deactivation process caused by coking during the oligomerization of ethene over H-ZSM-5. Zhu et al. applied the TEOM technique in a series of investigations concerning adsorption/desorption characteristics of light alkanes [33–36] and unsaturated C4 hydrocarbons [37] on silicalite-1 and DD3R zeolite, respectively, determined by both steady-state and transient uptake and desorption measurements.
TG and DTG studies revealed the formation of weak bonds between extraframework aluminum species in dealuminated H-ZSM-5 and acetylacetone applied for visualization of “NMR invisible” aluminum [38].
Thermal Analysis of Zeolites |
75 |
It is known that cations play a fundamental role in zeolite crystallization. Aiello et al. [39] used various thermoanalytical techniques to study the dehydration of aluminosilicate gel precursors containing Na+ or K+ ions. In the case of K+-containing gels one-step low-temperature dehydration was observed, while in the presence of Na+ ions a second peak appeared in the DTG and DSC curves at a temperature close to the dehydration temperature of the final zeolitic product. TG/DTG and DSC results confirmed the role of the sodium ion as a “structure-forming” cation and revealed that the presence of sodium ions in the synthesis mixtures induced, contrary to potassium, the formation of structural subunits or even more complex structures.
3
Chemical Modification of Zeolites Traced by Thermal Analysis
In only a few papers thermoanalytical methods were used to study the chemical modification of zeolites by gaseous reactants. The stoichiometry of the reaction between mordenite and phosgene was established by thermogravimetry [40].
Thermogravimetry combined with titration of the evolved HCl was applied to measure the temperature range of the solid-state ion exchange between H-zeolites and metal-chlorides and to determine the ion-exchange degree [41]. The simultaneous application of the two techniques made it possible to distinguish between changes in weight caused by desorption of physisorbed water and by solid-state reactions generating volatile product.
Besides the results of other techniques, TG/DTG data obtained during temperature-programmed reduction (TPR) of mixtures consisting of H-zeolite and Ga2 O3 or In2O3 provided additional evidence for the replacement of zeolitic protons by Ga+ or In+ ions in the so-called reductive solidstate ion exchange process [42–45]. The thermal analysis of a mixture of Ga2 O3 and H-ZSM-5 carried out after pretreatment in vacuum in a hydrogen flow revealed a relatively fast process accompanied by a weight loss at about 850 K [42]. In contrast, no change in the weight of the mixture was observed in an inert gas atmosphere. In H2, the reduction of Ga2O3 resulted in water, the evolution of which was monitored by TG. Results of XRD, XPS, and IR spectroscopy suggested that in the reductive thermal treatment gallium was transferred into the zeolite. Later, In2 O3 instead of Ga2O3 was mixed with the H-form of zeolites. These mixtures and simultaneously pure In2 O3 were subjected to temperature-programmed reduction in a H2 atmosphere. The temperatures at which weight losses and DTG peaks were observed due to the evolution of water as a reaction product differed significantly for In2 O3 in the pure form and for In2 O3/H-zeolite (e.g., H-Y, H-ZSM-5) mixtures [43]. The same phenomenon was reported in [44]. DTG curves are demonstrated in Fig. 5. The high-temperature DTG peak at about 730 K was attributed to the
76 |
G. Pál-Borbély |
Fig. 5 DTG curves registered during TPR of NH4 Na-Y/In2 O3 mixtures (NH4 /In corresponds to (a) 1, (b) 2 and (c) 3); (d) denotes In2 O3 and (e) a NaY/In2 O3 mixture (Na/In = 1) (reproduced from [44])
reduction of In2O3 to metallic indium. In contrast, the reduction was found to proceed in mixtures with H-zeolites at lower temperature (between 530 and 700 K) via In2 O according to the overall transformation
In2O3 + 2H+Z– + 2H2 –→ 2In+Z– + 3H2O . |
(1) |
The replacement of protons by In+ cations was evidenced by IR spectroscopy [44, 45]. It was shown that In+ cations once incorporated by RSSIE (involving hydrogen or the template as reducing agents) can be oxidized to InO+ and again reversibly reduced to In+, both species occupying cation sites during the redox process.
4
Thermal Removal and Decomposition of Templates Occluded in Zeolites
Thermal analysis was widely applied for characterization of zeolites. Undoubtedly, however, the main objects of thermal studies were zeolites containing organic compounds. Organic molecules may be chemisorbed on the Brönsted and Lewis sites of zeolites, physisorbed in their pore system, or entrapped in their channels and cavities during the synthesis.
