
Multiple Bonds Between Metal Atoms / 15-Extended Metal Atom Chains
.pdf
15
Extended Metal Atom Chains
John F. Berry,
Texas A&M University
15.1 Overview
The previous chapters of this book have shown that the chemistry of dinuclear compounds with metal–metal bonds is extensive. But why should this chemistry be limited to bonds between only two metal atoms? As will be seen in this chapter, it is not. By using expanded bridging ligands as in 15.1, it is possible to synthesize extended metal atom chain (EMAC) compounds. Such EMACs with polypyridylamido or related ligands will be the main subject of this chapter. Brief reviews on this subject have appeared.1
A |
B |
A |
B |
D |
|||||
C |
C |
E etc. |
|||||||
M |
|
4 |
|
|
|
|
|
|
4 |
|
|
|
|
|
|
||||
M |
M |
M |
M |
||||||
15.1
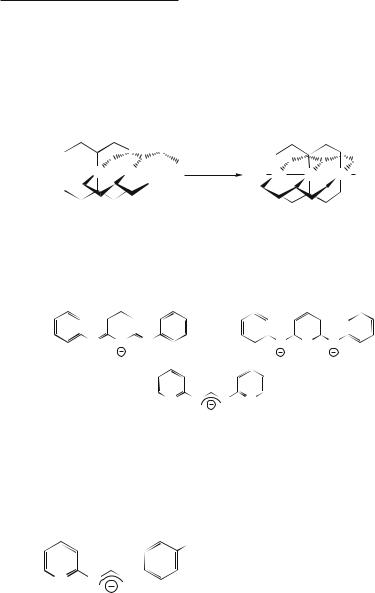
The first EMAC was synthesized serendipitously in 1968 by Hurley and Robinson and formulated as Ni3(dpa)4Cl2 (dpa is the anion of di-2-pyridylamine; see 15.2).2
N N N
di-2,2'-pyridylamide (dpa)
15.2
Though Hurley and Robinson were able to ascertain the trinickel formula Ni3(dpa)4Cl2 from careful elemental analysis, they postulated a structure which was shown in 1991 by X-ray crystallography3 to be incorrect. The correct structure, as determined by Aduldecha and Hathaway,3 is shown in Fig. 15.1a, and an end-on view along the Ni3 axis is shown in Fig. 15.1b. This compound contains only d8 Ni2+ ions and therefore Ni–Ni bonds are not expected, though subsequently, tricobalt,4 triruthenium,5 trirhodium,5 and trichromium6 complexes of dpa have been synthesized, all of which have metal–metal bonds.
669

670Multiple Bonds Between Metal Atoms Chapter 15
Fig. 15.1. The structure of Ni3(dpa)4Cl2 shown (a) perpendicular to the Ni3 axis, and
(b) along the Ni3 axis.
The type of structure in Fig. 15.1 is typical of the compounds discussed in this chapter. Four dpa anions wrap helically around the trimetal chain with a considerable torsion angle, typically ~50˚ from end to end. This torsion angle can be attributed to steric repulsions between opposite pyridyl hydrogen atoms as in 15.3.
H H
N N N
15.3
The metal ions are most often in the +2 oxidation state, though other oxidation states are known, and anionic ligands, usually in axial positions, are present in order to balance the charge. These range from simple halides (Cl, Br) to pseudohalides (CN, NCS) to more complex anions (C>CPh, Ag(CN)2). Neutral molecules such as water or acetonitrile are also known to occupy axial positions.
The dpa ligand is the shortest in a series of polypyridylamide ligands (see 15.4), which have been shown to stabilize linear arrays of five,7 seven,8 and even nine9 metal atoms in compounds with the general formula Mn(L)4X2 (for n = 5, L = tpda; n = 7, L = teptra; n = 9, L = peptea).† The synthetic methodology exists to produce ligands which can hold greater numbers of metal atoms.
The study of EMACs focuses primarily on their interesting physical properties. The magnetic properties of polypyridylamido complexes are of importance, since the large majority of these compounds contain unpaired electrons. For example, Co3(dpa)4Cl2 has been shown to undergo a thermally induced spin transition from a low-spin (S = ½) to a high-spin (S = 3/2 or 5/2) state.10 Also, the structural results are often complicated. Both Cr3(dpa)4Cl211 and Co3(dpa)4Cl210 have
†The nomenclature for these ligands follows from the number of pyridyl groups and amido groups of the ligand. The ligand shown in 15.4 which holds five metal atoms has three pyridyl groups and two amido groups and is thus called “tripyridyldiamide,” with the abbreviation tpda. The ligand teptra is thus “tetrapyridyltriamide,” and peptea is “pentapyridyltetraamide.”
Extended Metal Atom Chains 671
Berry
been shown to exist in crystalline polymorphs with drastically different metal-metal distances. In some forms, the two M–M distances of the compound are equivalent yielding a symmetrical D4 core structure. In other cases, the compounds are distinctly unsymmetrical (C4 symmetry) with a short M–M distance and a long M···M separation with ¨d(M–M) (i.e. the difference between the two independent M–M distances) as much as 0.18 Å (see 15.5). This phenomenon is relevant to Cr5 chains also.12
|
|
N |
N |
N |
N |
N |
|
|
|
|
|
tripyridyldiamide = tpda |
|
|
|||
|
N |
N |
N |
N |
N |
N |
N |
|
|
|
|
tetrapyridyltriamide = teptra |
|
|
|||
N |
N |
N |
N |
N |
N |
N |
N |
N |
pentapyridyltetraamide = peptea
15.4
X M M M X X M M
M X X M M

 M X
M X
s-M3(dpa)4X2 u-M3(dpa)4X2
15.5
Theoretical work on EMACs is important to the understanding of their properties. The molecular orbital (MO) theory of metal-metal bonds as presented in Chapter 1 is useful in describing the electronic structures of oligomeric chains. Calculations have been performed using DFT for Co3(dpa)4Cl213 and Cr3(dpa)4Cl214 in order to explain some of the experimental results. Also, qualitative MO diagrams for M3(dpa)4Cl215 and M5(tpda)4Cl216 have been proposed. At some point, as the EMACs become longer, the discrete molecular orbitals must be viewed as comprising continuous bands, and simple MO theory must give way to the band theory for a proper description of the electronic structure. Detailed descriptions of this are beyond the scope of this book, though it should be noted that band calculations on a hypothetical infinite chain of equally spaced Cr2+ atoms with a polypyridylamido backbone have been performed.17
The remainder of the chapter will discuss the synthetic, structural, physical, and theoretical work done for polypyridylamido complexes of chromium, cobalt, nickel, and second row metals. Other recent developments in the chemistry of EMACs will be discussed lastly.
15.2 EMACs of Chromium
The parent trichromium dipyridylamido compound, Cr3(dpa)4Cl2, is prepared in high yield in a straightforward reaction between excess CrCl2 and lithium dipyridylamide in THF.6,11 In
672Multiple Bonds Between Metal Atoms Chapter 15
the early stages of the reaction, red quadruply-bonded Cr2(dpa)4 is formed, which is then converted to the green Cr3(dpa)4Cl2 upon heating to reflux as shown in 15.6. A ligand shuffling process is proposed in this transformation of the ligands from a trans-2:2 geometry in Cr2(dpa)4 to the µ3 bridging mode in the trinuclear species.18 Such a ligand shuffling process has recently been studied by variable temperature NMR spectroscopy for a dichromium complex with multidentate ligands.19
Cr Cr CrCl2
Cr CrCl2
THF, heat
Cl Cr Cr Cr Cl
Cr Cl
15.6
This synthetic method is also employed in the preparation of trichromium complexes of the ligands DPhIP20 (di(phenylimino)piperidinate), BPAP21 (2,6-bisphenylaminopyridinate), and DPyF22 (dipyridylformamidinate), shown schematically in 15.7.
N N N |
N N N |
DPhIP |
BPAP |
|
H |
N |
N N N |
|
DPyF |
|
15.7 |
Unsymmetrical amidinate ligands have also been used, with various substituents on the aryl rings, as in 15.8.23
R3 |
|
|
|
PhPyF: R1 = R2 = R3 = H |
|
|
|
PhPyBz: R1 = Ph, R2 = R3 = H |
|
|
|
|
R2 |
|
|
|
R1 |
AniPyF: R1 = R3 = H, R2 = OMe |
|
|
|
|
||
N |
N |
N |
|
TolPyF: R1 = R3 = H, R2 = Me |
|
FPhPyF: R1 = R3 = H, R2 = F |
PhPcF: R1 = R2 = H, R3 = Me
15.8
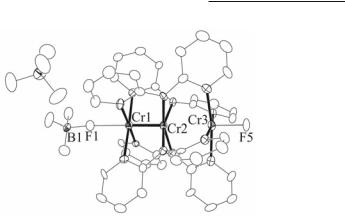
Replacement of one of the axial Cl ions by a BF46 or PF611 anion is achieved by metathesis with one equivalent of the corresponding silver reagent, but reaction with more than one equivalent of silver causes oxidation of the Cr36+ unit to Cr37+.24 For example, reaction of Cr3(dpa)4Cl2 with two equivalents of AgBF4 yields the oxidized complex [Cr3(dpa)4F(BF4)]BF4 shown in Fig. 15.2. The axial Cl ions can also be replaced by phenylacetylide by reaction of Cr3(dpa)4Cl2 with LiC>CPh.11,18 This reaction was difficult to control, and pure products were not obtained. Recently, Cr3(dpa)4(CCPh)2 has been prepared in good yields and excellent purity by using [Cr3(dpa)4(NCCH3)2](PF6)2 as starting material.25
Extended Metal Atom Chains 673
Berry
Fig. 15.2. Structure of [Cr3(dpa)4F(BF4)]BF4.
By far the most facile reactions are oxidations. The parent compound Cr3(dpa)4Cl2 has a reversible one-electron oxidation wave at E½ = 74 mV vs Ag/AgCl11 and thus mild oxidants such as ferrocenium salts react to convert Cr3(dpa)4Cl2 to the corresponding cation [Cr3(dpa)4Cl2]+.24
Cr3(dpa)4Cl2 + [Cp2Fe]X Α [Cr3(dpa)4Cl2]X + Cp2Fe
Structurally characterized Cr36+ and Cr37+ compounds are listed in Table 15.1. The variability of the Cr–Cr bond lengths in these structures is compounded by the fact that the Cr3 chains can exist in symmetric (D4) or unsymmetric (C4) forms. The question of whether a Cr36+ chain is symmetrical or unsymmetrical appears to have its answer in the properties of the bridging and axial ligands. For Cr3(dpa)4Cl2, all of the known structures contain unsymmetrical molecules, though replacement of the chloride ligands by cyanide ligands results in a symmetrical complex of D4 symmetry.26 Complexes of the unsymmetrical amidinates also form symmetrical chains.23,27 Trichromium complexes of the ligands DPyF,22 DPhIP,20 and BPAP21 are all unsymmetrical, however, each with a very short Cr–Cr quadruple bond (< 2.0 Å) and a long distance to the isolated Cr(II) species (2.59 to 2.74 Å). Complexes with an unsymmetrical set of axial ligands, or no axial ligands at all, are in all cases unsymmetrical. Furthermore, all known Cr37+ compounds have unsymmetrical chains with short Cr24+ quadruple bonds and long distances to the isolated Cr3+ ions.24
All of the known trichromium compounds are paramagnetic. Variable temperature magnetic susceptibility data for both symmetrical and unsymmetrical Cr36+ compounds follow the Curie law with µeff = 4.6 - 5.1 µB corresponding to four unpaired electrons.11 Thus, it is not possible to distinguish between symmetrical and unsymmetrical compounds by magnetic susceptibility data alone. For the unsymmetrical Cr36+ compounds, the four unpaired electrons are thought to be localized on the isolated high spin Cr2+ ion (since the quadruply bonded Cr24+ unit is diamagnetic). In the case of the symmetrical Cr36+ species, the four unpaired electrons are thought to be delocalized over the Cr3 chain. A qualitative MO scheme for the symmetrical Cr3 compounds has been presented to account for this15 and it is shown in 15.9a. Since the Cr–Cr distances are fairly long, the β interactions of the dxy orbitals are neglected. Thus, the β orbitals and the / nonbonding orbitals are essentially degenerate, and using Hund’s rule to fill in the 12 electrons for a Cr36+ unit, the ground state therefore has S = 2.

Table 15.1. Structural data for trimetal EMACs
Cr36+ Compounds
Compound |
Space |
Cr1–Cr2, Å |
Cr2–Cr3, Å |
µeff, µB |
Remarka |
ref. |
|
Group |
|||||||
|
|
|
|
|
|
||
Cr3(dpa)4Cl2·CH2Cl2 |
Pnn2 |
2.254(4) |
2.477(4) |
5.1 |
U |
26c |
|
Cr3(dpa)4Cl2·C6H6 |
Pna21 |
2.227[9], 2.236[9]b |
2.483[9], 2.481[9]b |
NR |
U |
26c |
|
Cr3(dpa)4Cl2·C7H8 |
Pca21 |
2.24[1]e |
2.48[1]e |
NR |
U |
26c |
|
Cr3(dpa)4Cl2·THF |
– |
|
|
|
|
|
|
P4n2 |
2.365(2)d |
2.365(2)d |
NR |
S |
11d |
||
Cr3(dpa)4Cl(BF4)·CH2Cl2 |
C2/c |
1.9952(8) |
2.6427(8) |
3.29 |
U |
6 |
|
Cr3(dpa)4Cl(PF6)·2CH2Cl2 |
C2/c |
2.008(1) |
2.614(1) |
4.62 |
U |
11 |
|
Cr3(dpa)4(NCS)2·2C2H4Cl2 |
P21/c |
2.277(2) |
2.391(2) |
NR |
U |
67 |
|
Cr3(dpa)4(C>CPh)2f |
P21/c |
2.415(2) |
2.422(2) |
NR |
S |
11 |
|
[Cr3(DPhIP)4Cl]Cl·1.5CH2Cl2·0.5H2O |
P21/c |
1.932(2) |
2.659(2) |
NR |
U |
20 |
|
[Cr3(DPhIP)4(NCMe)](PF6)2·H2O·4CH3CN |
P4/n |
1.907(2) |
2.633(2) |
4.3 |
U |
20 |
|
(NBu4)2[Cr3(BPAP)4]·THF |
C2/c |
1.904(3) |
2.589(2) |
NR |
U |
21 |
|
Cr3(PhPyBz)4Cl2·1.64CH2Cl2·0.52hexane·0.42THF |
– |
|
|
|
|
|
|
P1 |
2.269(1) |
2.513(1) |
5.3 |
U |
23 |
||
Cr3(PhPyF)4Cl2·CH2Cl2 |
P43212 |
2.4380(8) |
2.4602(8) |
4.78(2) |
S |
27 |
|
Cr3(AniPyF)4Cl2 |
P21/c |
2.4789(7) |
2.4759(7) |
4.69(1) |
S |
27 |
|
Cr3(TolPyF)4Cl2·2H2O |
Pccn |
2.4298(9) |
2.4298(9) |
NR |
S |
27 |
|
Cr3(FPhPyF)4Cl2 |
– |
|
|
|
|
|
|
P1 |
2.460(1) |
2.500(1) |
NR |
S |
27 |
||
Cr3(PhPcF)4Cl2·THF·0.5hexane |
C2/c |
2.4743(8) |
2.4743(8) |
4.65(2) |
S |
27 |
|
Cr3(PhPcF)4Cl2·0.61Et2O |
P2/n |
2.216(1) |
2.646(1) |
4.70(1) |
U |
27 |
|
674 |
|
15 Chapter |
|
Bonds Multiple |
|
||
|
|
Atoms Metal Between |

Cr37+ Compounds, All Are Unsymmetrical
|
|
|
|
|
|
|
Compound |
|
Space |
Cr–Cr, Å |
Cr···Cr, Å |
|
µeff, µB |
ref. |
|||
|
|
|
|
|
|
|
|
Group |
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
[Cr3(dpa)4Cl2]Cl·2CH2Cl2·THF |
|
Ibca |
2.12(1)e |
2.47(1)e |
3.85(5) |
|
24 |
||||||||||
[Cr3(dpa)4Cl2]AlCl4·CH2Cl2 |
|
P21/n |
2.010(1) |
2.555(1) |
3.85(5) |
|
24 |
||||||||||
[Cr3(dpa)4Cl2]FeCl4·CH2Cl2 |
|
P21/n |
2.009(1) |
2.562(1) |
NR |
|
24 |
||||||||||
[Cr3(dpa)4Cl2]I3·THF·2H2O |
|
P21/c |
2.08(1), 2.09(2)e |
2.49(1), 2.48(2)e |
NR |
|
24 |
||||||||||
[Cr |
3 |
(dpa) |
4 |
Cl |
2 |
]PF |
·2CH Cl |
2 |
|
P2 |
1 |
/n |
2.09(2), 2.09(2)e |
2.48(2), 2.48(2)e |
NR |
|
24 |
|
|
|
6 |
2 |
|
|
|
|
|
|
|
|
|||||
[Cr3(dpa)4F(BF4)]BF4·2CH2Cl2·C6H14 |
|
Pna21 |
1.900(2), 1.906(2)b |
2.596(3), 2.579(3)b |
4.4 |
|
6 |
||||||||||
[Cr3(dpa)4ClF]BF4·CH2Cl2·C6H14 |
|
P21/n |
2.039(5), 2.066(9)e |
2.507(4), 2.491(9)e |
NR |
|
24 |
||||||||||
[Cr3(DPhIP)4F(NCMe)](BF4)2·5MeCN |
|
P4/n |
1.968(2) |
2.594(2) |
NR |
|
20 |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
Crystal Structures of Co3(dpa)4Cl2 |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
Interstitial Molecules |
T, K |
Space |
Co1–Co2 |
Co2–Co3 |
µeff, µB |
Remarka |
ref. |
||||||||
|
|
Group |
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
2CH2Cl2·H2O |
|
|
|
I4g |
|
2.290(3) |
2.472(3) |
2.6 |
U |
4 |
|||||||
Co(dpa)2 |
|
|
|
|
|
|
|
P4/n |
2.285(1) |
2.459(1) |
NR |
U |
31 |
||||
CH2Cl2 |
|
|
|
|
|
|
296 |
Pnn2 |
2.3369(4) |
2.3369(4) |
2.9 |
S |
10 |
||||
|
|
|
|
|
|
|
|
|
168 |
Pnn2 |
2.3178(9) |
2.3178(9) |
NR |
S |
31,32 |
||
|
|
|
|
|
|
|
|
|
109 |
Pn |
|
|
2.3224(8) |
2.3214(8) |
NR |
S |
10 |
|
|
|
|
|
|
|
|
|
20 |
Pn |
|
|
2.34(1) |
2.34(1) |
NR |
S |
10 |
2CH2Cl2 |
|
|
|
|
|
|
|
– |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
298 |
I4 |
|
|
2.299(1) |
2.471(1) |
4.4 |
U |
10 |
|||
|
|
|
|
|
|
|
|
|
213 |
– |
|
|
2.294(1) |
2.466(1) |
NR |
U |
39 |
|
|
|
|
|
|
|
|
|
I4 |
|
|
||||||
|
|
|
|
|
|
|
|
|
173 |
– |
|
|
2.2958(9) |
2.457(1) |
NR |
U |
10 |
|
|
|
|
|
|
|
|
|
I4 |
|
|
||||||
|
|
|
|
|
|
|
|
|
133 |
– |
|
|
2.295(1) |
2.440(1) |
NR |
U |
10 |
|
|
|
|
|
|
|
|
|
I4 |
|
|
||||||
|
|
|
|
|
|
|
|
|
20 |
– |
|
|
2.3035(7) |
2.3847(8) |
NR |
U |
10 |
|
|
|
|
|
|
|
|
|
I4 |
|
|
||||||
Berry |
Chains Atom Metal Extended |
675
Interstitial Molecules |
T, K |
Space |
Co1–Co2 |
Co2–Co3 |
µeff, µB |
Remarka |
ref. |
|
Group |
||||||||
|
|
|
|
|
|
|
||
0.85Et2O·0.15CH2Cl2 |
296 |
P21/c |
2.3230(3) |
2.3667(4) |
3.5 |
S |
40 |
|
|
213 |
P21/c |
2.3193(3) |
2.3352(3) |
NR |
S |
40 |
|
|
120 |
P21/c |
2.3191(3) |
2.3304(3) |
NR |
S |
40 |
|
THF |
295 |
Pccn |
2.3484(4) |
2.4234(8) |
4 |
sl. U |
40 |
|
|
120 |
Pccn |
2.3111(4) |
2.4402(7) |
NR |
U |
40 |
|
cyclohexane |
295 |
Pccn |
2.3620(5) |
2.3620(5) |
4 |
S |
40 |
|
|
213 |
Pccn |
2.3311(5) |
2.3311(5) |
NR |
S |
40 |
|
|
120 |
P21/c |
2.3127(5) |
2.3253(5) |
NR |
S |
40 |
|
benzene |
316 |
Pca21 |
2.3417(9) |
2.3665(9) |
4.2 |
S |
40 |
|
|
260 |
Pna21 |
2.324(1), 2.323(1)b |
2.350(1), 2.346(1)b |
NR |
S |
40 |
|
|
213 |
Pna21 |
2.323(1), 2.326(2)b |
2.344(2), 2.338(2)b |
NR |
S |
40 |
|
|
170 |
Pna21 |
2.3135(8), 2.3189(8)b |
2.3280(8), 2.3283(8)b |
NR |
S |
40 |
|
1.75toluene·0.5hexane |
298 |
– |
2.310(2), |
2.471(2), |
4.25 |
U |
40 |
|
P1 |
||||||||
|
|
|
2.312(2)b |
2.442(2)b |
|
U |
|
|
|
170 |
– |
2.3046(6), |
2.4216(6), |
NR |
U |
40 |
|
|
P1 |
|||||||
|
|
|
2.3084(6)b |
2.3622(6)b |
|
sl. U |
|
|
|
110 |
– |
2.3135(6), |
2.3728(6), |
NR |
sl. U |
40 |
|
|
P1 |
|||||||
|
|
|
2.3174(6)b |
2.3245(6)b |
|
S |
|
|
|
90 |
– |
2.3098(6), |
2.3660(6), |
NR |
sl. U |
40 |
|
|
P1 |
|||||||
|
|
|
2.3139(6)b |
2.3196(6)b |
|
S |
|
|
|
|
|
|
|
|
|
||
|
|
Other Co36+ Compounds |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Compound |
T, K |
Space |
Co1–Co2 |
Co2–Co3 |
µeff, µB |
Remarka |
ref. |
|
Group |
||||||||
|
|
|
|
|
|
|
||
Co3(dpa)4Cl(BF4)·2CH2Cl2 |
|
C2/c |
2.277(2) |
2.504(2) |
NR |
U |
32 |
|
Co3(dpa)4(BF4)2·2CH2Cl2 |
|
P21/c |
2.254(2) |
2.252(2) |
NR |
S |
32 |
|
|
676 |
|
15 Chapter |
|
Bonds Multiple |
|
||
|
|
Atoms Metal Between |
|
|
|
Compound |
T, K |
Space |
Co1–Co2 |
Co2–Co3 |
µeff, µB |
Remarka |
ref. |
|||||
|
|
|
Group |
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Co3(dpa)4(CN)2·CH2Cl2 |
|
|
Pnn2 |
2.3392(2) |
2.3392(2) |
2.1 |
S |
35 |
|||||||
Co3(dpa)4(NCS)2·1.5CH2Cl2 |
|
P21/c |
2.3223(6) |
2.3087(6) |
2.5 |
S |
35 |
||||||||
Co |
(dpa) |
|
(NCS) |
|
·2CH |
|
Cl |
|
|
– |
2.300(2)b |
2.344(2)b |
NR |
sl. U |
67 |
4 |
2 |
2 |
2 |
|
P1 |
||||||||||
3 |
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
2.311(2)b |
2.324(2)b |
|
S |
|
Co3(dpa)4(NCS)2·5THF |
|
|
– |
|
|
|
|
|
|||||||
|
|
P1 |
2.313(2) |
2.309(2) |
NR |
S |
35 |
||||||||
Co3(dpa)4(NCS)2·2toluene |
|
Fdd2 |
2.3140(8) |
2.3140(8) |
NR |
S |
35 |
||||||||
Co3(dpa)4(NCNCN)2·2CH2Cl2 |
|
P21/c |
2.3194(8) |
2.3184(8) |
2.2 |
S |
35 |
||||||||
[Co3(dpa)4(NCMe)2](PF6)2·3MeCN |
|
– |
|
|
|
|
|
||||||||
|
P1 |
2.301(1) |
2.304(1) |
2.25 |
S |
33 |
|||||||||
[Co3(dpa)4(NCMe)2](PF6)2·MeCN·2Et2O |
213 |
P21 |
2.300(1) |
2.298(1) |
NR |
S |
33 |
||||||||
|
|
|
|
|
|
|
|
|
163 |
P21 |
2.301(1) |
2.299(1) |
NR |
S |
33 |
Co3(dpa)4Br2·CH2Cl2 |
|
|
|
240 |
Pnn2 |
2.3234(6) |
2.3234(6) |
2.7 |
S |
34 |
|||||
|
|
|
|
|
|
|
|
|
147 |
Pnn2 |
2.3182(8) |
2.3182(8) |
NR |
S |
34 |
|
|
|
|
|
|
|
|
|
111 |
Pnn2 |
2.3164(8) |
2.3164(8) |
NR |
S |
34 |
Co3(dpa)4Br2·cyclohexane |
298 |
P2/n |
2.3830(3) |
2.3830(3) |
NR |
S |
34 |
||||||||
|
|
|
|
|
|
|
|
|
213 |
P2/n |
2.3566(3) |
2.3566(3) |
NR |
S |
34 |
|
|
|
|
|
|
|
|
|
150 |
P2/n |
2.3262(3) |
2.3262(3) |
NR |
S |
34 |
|
|
|
|
|
|
|
|
|
110 |
P2/n |
2.3188(2) |
2.3188(2) |
NR |
S |
34 |
Co3(dpa)4Br2·1.75toluene·0.5hexane |
|
– |
|
|
|
|
|
||||||||
295 |
P1 |
2.312(1), |
2.469(1), |
NR |
U |
34 |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
2.323(1)b |
2.433(1)b |
|
U |
|
|
|
|
|
|
|
|
|
|
213 |
– |
2.305(1), |
2.451(1), |
NR |
U |
34 |
|
|
|
|
|
|
|
|
|
P1 |
||||||
|
|
|
|
|
|
|
|
|
|
|
2.3099(9)b |
2.392(1)b |
|
sl. U |
|
|
|
|
|
|
|
|
|
|
170 |
– |
2.3062(9), |
2.4313(9), |
NR |
U |
34 |
|
|
|
|
|
|
|
|
|
P1 |
||||||
|
|
|
|
|
|
|
|
|
|
|
2.3118(8)b |
2.3536(9)b |
|
sl. U |
|
|
|
|
|
|
|
|
|
|
110 |
– |
2.3097(9), |
2.3892(9), |
NR |
sl. U |
34 |
|
|
|
|
|
|
|
|
|
P1 |
||||||
|
|
|
|
|
|
|
|
|
|
|
2.3173(8)b |
2.3162(8)b |
|
S |
|
Berry |
|
Chains Atom Metal Extended |
|
677 |
|

Compound |
T, K |
Space |
Co1–Co2 |
Co2–Co3 |
µeff, µB |
Remarka |
ref. |
Group |
|||||||
Co3(depa)4Cl2 |
|
– |
|
|
|
|
|
213 |
P4n2 |
2.3611(7) |
2.3611(7) |
NR |
S |
36 |
|
Co3(depa)4Cl2·0.5hexane |
|
– |
|
|
|
|
|
213 |
P4n2 |
2.3609(5) |
2.3609(5) |
NR |
S |
36 |
|
|
297 |
– |
2.3787(7) |
2.3787(7) |
4.9 |
S |
36 |
|
P4n2 |
||||||
Co3(depa)4Cl2·acetone |
|
– |
|
|
|
|
|
213 |
P4n2 |
2.352(1) |
2.352(1) |
NR |
S |
36 |
|
Co3(depa)4Cl2·4CH2Cl2·2H2O |
|
– |
|
|
|
|
|
213 |
I4c2 |
2.3309(8) |
2.3309(8) |
NR |
S |
36 |
|
Co3(depa)4(CN)2·0.5hexane |
|
– |
|
|
|
|
|
213 |
P4n2 |
2.3371(4) |
2.3371(4) |
2.7 |
S |
36 |
|
Co3(depa)4(CN)2·4CH2Cl2·2H2O |
|
– |
|
|
|
|
|
213 |
I4c2 |
2.3357(7) |
2.3357(7) |
NR |
S |
36 |
|
|
|
|
|
|
|
|
|
|
|
|
Co37+ Compounds |
|
|
|
|
|
|
|
|
|
|
|
|
[Co3(dpa)4Cl2]BF4·xCH2Cl2 |
300 |
P21/n |
2.325(1) |
2.341(1) |
2.5 |
S |
37 |
|
213 |
P21/n |
2.321(1) |
2.327(1) |
NR |
S |
37 |
|
100 |
P21/n |
2.3168(8) |
2.3289(8) |
NR |
S |
37 |
|
|
|
|
|
|
|
|
|
|
|
Ni36+ Compounds |
|
|
|
|
|
|
|
|
|
|
|
|
Compound |
|
Space |
Ni···Ni, Å |
µeff, µB |
ref. |
||
|
Group |
||||||
|
|
|
|
|
|
|
|
Ni3(dpa)4Cl2·0.23H2O·0.5(CH3)2CO |
|
C2/c |
2.443(1), 2.443(1); 2.431(1)h |
3.5 |
|
3 |
|
Ni3(dpa)4Cl2·2CH2Cl2 |
|
– |
|
|
|
|
|
|
I4 |
2.4386(9), 2.422(1) |
|
2.8 |
|
47 |
|
Ni3(dpa)4Cl2·THF |
|
Pccn |
2.4172(8) |
|
NR |
|
47 |
Ni3(dpa)4Cl2·Et2O |
|
P21/c |
2.438(1), 2.433(1) |
|
NR |
|
15 |
Ni3(dpa)4Cl2·2toluene·0.5hexane |
|
– |
|
|
|
|
|
|
P1 |
2.4249(9), 2.4253(9); |
|
NR |
|
15 |
|
|
|
|
2.4265(9), 2.4386(9)b |
|
|
|
|
Ni3(dpa)4(NO3)2 |
|
P212121 |
2.3982(5), 2.4074(5) |
|
NR |
|
52 |
Ni3(dpa)4(N3)2 |
|
P21/c |
2.4325(7), 2.4356(7) |
|
2.7 |
|
53 |
|
678 |
|
15 Chapter |
|
Bonds Multiple |
|
||
|
|
Atoms Metal Between |
