
Multiple Bonds Between Metal Atoms / 14-Nickel, Palladium and Platinum Compounds
.pdf
14
Nickel, Palladium and
Platinum Compounds
Carlos A. Murillo,
Texas A&M University
14.1 General Remarks
The most common oxidation state for the group 10 elements is two which gives rise to the very stable d8 electronic configuration. For Pd and Pt (and to a lesser extent Ni), the stereochemistry of such d8 species is dominated by square planar compounds. The tetravalent state is often found in Pd and Pt compounds but all of them are mononuclear or without metal-metal bonds when they associate. Whenever bridging ligands couple divalent square planar species, compounds of the paddlewheel type form, but these again are devoid of metal-to-metal bonds as all bonding and antibonding MOs are occupied giving a μ2/4β2β*2/*4μ*2 configuration with a net bond order of zero. Thus, only when the d8 electronic configuration is altered, i.e., by oxidation of the metal centers, can metal-metal bond formation occur. Therefore, a one-electron oxidation of a non-bonded paddlewheel complex would be expected to yield a paramagnetic species with an M25+ core and an electronic configuration μ2/4β2β*2/*4μ* (or a variation thereof) with a bond order of 0.5. A 2-electron oxidation would be expected to give a diamagnetic M26+ core with an electronic configuration of μ2/4β2β*2/*4 and a single M–M bond. Since higher oxidation states are typically favored for the heavier elements of a given group of the periodic table, Pt would be expected to be most readily oxidized. Indeed, almost all of the M26+ complexes in this group contain singly-bonded Pt26+ units. In this chapter we will focus our attention primarily to those dimetal compounds in which each metal atom unit possesses a square planar configuration and two square planes (with or without additional axial ligands) parallel to each other.
A useful review of quadruply bridged dinuclear complexes of platinum, palladium and nickel has appeared.1 There are also several reviews covering various aspects of paddlewheel Pt chemistry. These will be referenced, as appropriate, later on.
14.2 Dinickel Compounds
To date, no authentic singly bonded dinickel(III) complex has been isolated in the solid state and characterized, although several closely related dinickel(II) species without metalmetal bonds have been prepared and various attempts made to oxidize them. The dithioacetato complex Ni2(S2CCH3)4, in which the Ni···Ni separation is 2.564(1) Å, has been oxidized2 to [Ni2(S2CCH3)4I] which consists of linear chains of ···I···[Ni2S8]···I···[Ni2S8]··· with Ni–Ni and
633

634Multiple Bonds Between Metal Atoms Chapter 14
Ni–I distances of 2.514(3) Å and c. 2.93 Å, respectively. Within the individual Ni2S8 units the torsional angle is 28˚. This compound is said to be EPR-silent. This is in contrast to the EPR-active [Ni2(DTolF)4]+ cation, where DTolF = [(p-tol)NCHN(p-tol)]-, which can be generated by the electrochemical oxidation of Ni2(DTolF)4 (E1/2(ox) = +0.73 V in Bun4NPF6/CH2Cl2 versus Ag/AgCl).3,4 This spectrum is consistent with axial symmetry, with g = 2.210 and g = 2.038. Thus the oxidation can be considered as metal centered. The dark-green complex [Ni2(DTolF)4]BF4 has been prepared4 by oxidizing Ni2(DTolF)4 with [Ag(NCCH3)2]BF4 in dichloromethane. The Ni–Ni distance in the Ni25+ complex (2.418(4) Å) is appreciably shorter than that in Ni2(DTolF)4(H2O)2 (2.485(2) Å) while the torsional angle is larger (27.4˚ versus 16.85˚). A SCF-X_-SW calculation on the model species Ni2(HNCHNH)4 and [Ni2(HNCHNH)4]+, along with the EPR spectral results indicates that the electron is lost from an orbital with partial metal-based β* character, thereby explaining the Ni–Ni bond shortening upon oxidation. Interestingly, the electrochemical oxidation of [Ni2(DTolF)4]+ to
[Ni2(DTolF)4]2+ occurs at Ep,a = +1.25 V versus Ag/AgCl,3,4 but the dication is not stable and this process is irreversible.
14.3 Dipalladium Compounds
The number of compounds of PdIII that have been isolated and characterized is very limited. There is no aqua ion nor any classical anionic complexes, PdX63-; the latter have been made only in the solid state under harsh conditions.5 There are no binary compounds (“PdF3” is a mixed PdII, PdIV compound).6 In 1987 the compound (H3O)[PdL2](ClO4)4·3H2O, L = 1,4,7-trithiacy- clononane, was reported with a structure determination.7
14.3.1 A singly bonded Pd26+ species
The only authentic paddlewheel compound having a Pd26+ core is Pd2(hpp)4Cl2. Here, hpp is the anion of the guanidinate derivative 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine depicted in 14.1. This was prepared in low yield by oxidation of the non-metal–metal-bonded PdII complex Pd2(hpp)4 with PhICl2, NOPF6 or NOBF4 but always as a mixture of compounds.8 Hand-picked crystals are quite stable, even in air. The paddlewheel structure shown in Fig. 14.1 is centrosymmetric with the chloride ions occupying axial positions of the paddlewheel. Except for the absence of chlorine atoms in the precursor, the structures are similar. However, the Pd–Pd distance in the Pd26+ compound of 2.391(1) Å is considerably shorter by 0.164 Å than that of the precursor and 0.36 Å shorter than that in Pd metal itself. The Pd–Cl distance is 2.474(4) Å and the crystallographically unique Pd–N distance is 2.034(6) Å. A cyclic voltammogram of the precursor Pd2(hpp)4 in CH2Cl2 showed a wave at -0.12 V vs. Ag/AgCl and another less reversible wave at +0.82 V.
N
N  N
N
14.1
Geometry optimization by the Hartree-Fock self-consistent field method has shown excellent agreement between the calculated and experimental results with a Pd–Pd distance of 2.402 vs the observed 2.391 Å and a torsion angle of 22.6˚ vs the experimental value of 24˚. The HOMO/LUMO gap is calculated to be 167 kcal mol-1. The calculations indicate that the Pd–Pd bond is a μ-bond formed mainly by dz2–dz2 overlap. The electronic configuration to be assigned to the dipalladium core appears to be /4β2β*2/*4μ2. This is shown in Fig. 14.2.
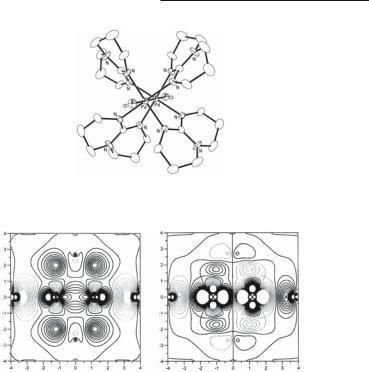
Nickel, Palladium and Platinum Compounds 635
Murillo
Fig. 14.1. The structure of the singly-bonded guanidinate compound Pd2(hpp)4Cl2.
Fig. 14.2. Contours of the HOMO (left) and LUMO (right) of the molecule Pd2(hpp)4Cl2 showing the Pd–Pd single bond on the plane formed by the Cl–Pd–Pd–Cl unit and four nitrogen atoms from two of the guanidinate ligands hpp. Positive and negative contours are in heavy and light lines, respectively. Both are antibonding with respect to the Pd–Cl interaction.
14.3.2 Chemistry of Pd25+ and similar species
The redox chemistry of the dipalladium(II) complex Pd2(DTolF)4, which is analogous to the nickel complex described in Section 14.2, has also been investigated.3,4 The cyclic voltammogram of solutions of this compound in Bun4NPF6/CH2Cl2 shows reversible one-electron oxidations at E1/2 = +0.81 V and +1.19 V versus Ag/AgCl, but only the one-electron oxidized product [Pd2(DTolF)4]PF6 has been prepared and structurally characterized.4 For this paramagnetic species the EPR spectrum suggests that the odd electron occupies a ligand-based molecular orbital,4 but SCF-X_-SW calculations show that the odd electron is located on an orbital with very significant metal character.4 Furthermore, it has been reported9 that the complex Pd2(DPhBz)4, where DPhBz = [PhNC(Ph)NPh]-, undergoes a reversible one-electron oxidation at +0.65 V to form the radical cation [Pd2(DPhBz)4]+. This shows an axially symmetric EPR signal at g = 2.17 and g = 1.98 that has been interpreted,9 in terms of the oxidation being metal-centered. The dipalladium(II) complex with 2-mercaptopyridine, Pd2(pyS)4, undergoes10 an irreversible one-electron oxidation at Ep,a = +0.61 V versus Ag/Ag+ in Bun4NClO4/CH2Cl2, but in the presence of halide ion (Cl- or Br-) a new quasi-reversible two-electron couple appears
at a much more negative potential (E1/2 = +0.13 V for Cl- and +0.15 V for Br-). This has been attributed10 to the generation of the dipalladium(III) species [Pd2(pyS)4X]+, but such a entity
has not been isolated or characterized further.
636Multiple Bonds Between Metal Atoms Chapter 14
14.3.3 Other compounds with Pd–Pd interactions
There are compounds with Pd–Pd interactions e.g., those in which two square planar PdII units are held together by bridging ligands such as the precursor to Pd2(hpp)4Cl2 in Section 14.3.1. However, these compounds do not have a formal metal-metal bond. Compounds with chains of three and four Pd atoms have also been made.11 The chains are cationic having BF4 or B(3,5-(CF3)2C6H3)4 counteranions. The Pdn chains are sandwiched between two all-trans-1,8- diphenyl-1,3,4,7-octatetraene molecules (DPOT). In 14.2, the Pd–Pd–Pd–Pd skeleton is essentially linear with the outer Pd···Pd separations of 2.7322(8) and the inner one of 2.654(1) Å. The overall formal oxidation number for the Pd4 unit is +2, Pd+0.5. Since molecules of this type do not fall in the category of metal–metal bonded species with a paddlewheel structure, no further comments will be made.
 2+
2+
Pd Pd Pd Pd Pd
14.2
14.4 Diplatinum Compounds
Singly bonded diplatinum complexes having Pt26+ cores are second only to dirhodium(II) species in the number of compounds that possess the μ2/4β2β*2/*4 configuration. With the exception of a few mononuclear species such as Bun4N[Pt(C6Cl5)4]12,13 and [Pt(1,4,7-trithiacy- clononane)2]3+,14 the vast majority of PtIII complexes are those that possess a Pt26+ core. Those with bridging oxyanions (sulfate, acetate, phosphate and pyrophosphite) and mixed N–O donor sets (such as hydroxypyridinato) predominate. For historical reasons we shall discuss the oxyanion-containing systems first. Several reviews have appeared covering diverse aspects of the chemistry of species containing PtIII units such as the chemistry of quadruply bridged dinuclear complexes of platinum, palladium and nickel,1 the preparation and properties of paddlewheel complexes,15 sulfate complexes,16 pyrophosphite complexes,17-19 complexes of 2-pyridone and its derivatives.20 There are also a series of reviews on chains such as those in the so-called platinum blue compounds and related species which also discuss chemistry relevant to complexes with a Pt26+ core.21-24 Assignment of electronic spectra of Pt26+ complexes will be discussed in Chapter 16. Some relevant papers should be consulted.25-28 Structural data for the Pt–Pt bonded complexes are given in Table 14.1.
Table 14.1. Structural data for diplatinum(III) compounds
|
|
|
|
|
|
Compounda |
r(Pt–Pt) (Å) |
r(Pt–L) (Å)b |
Donor |
ref. |
|
|
|
|
|
|
atom(s) L |
||||
|
|
|
|
|
|
|
|
|
|
|
K2[Pt2(SO4)4(H2O)2] |
|
2.461(1) |
2.111(7) |
O |
32 |
|||||
K2[Pt2(SO4)4(DMSO)2] |
2.471(1) |
2.126(6) |
O |
33 |
||||||
[Pt(pydz)4][Pt2(SO4)4(pydz)2]·2H2O |
2.482(1) |
2.140(6) |
N |
35 |
||||||
(pyH)2[Pt2(SO4)4(py)2] |
2.489(1) |
2.15(1) |
N |
34 |
||||||
Na2[Pt2(HPO4)4(H2O)2] |
2.486[2]c |
2.15[1]c |
O |
32,41 |
||||||
(3,4-Me2C5H3NH)2[Pt2(HPO4)4(3,4-Me2C5H3N)2] |
2.494(1) |
2.16(1) |
N |
42 |
||||||
(Ph4As)2[Pt2(HPO4)4(THT)2]·2H3PO4 |
2.525(1) |
2.462(1) |
S |
43 |
||||||
(pyH)[Pt2(H2PO4)(HPO4)3(py)2]·H2O |
2.494(1) |
2.14[4] |
N |
40 |
||||||
(Et4N)2[Pt2(H2PO4)2(HPO4)2Cl2]·H2O |
2.529(1) |
2.448(4) |
Cl |
32 |
||||||
Na10[Pt2(PO4)4(gu)2]·22H2O |
2.534(1) |
2.141(2) |
N |
46 |
||||||
K4[Pt2(pop)4Cl2]·2H2O |
2.695(1) |
2.407(2) |
Cl |
54,61 |
||||||
K4[Pt2(pop)4(CH3)I]·2H2O |
2.782(1) |
2.18(2) |
C |
58 |
||||||
|
|
|
|
|
|
|
|
2.816(3) |
I |
|
|
|
|
|
|
||||||
(Bun4N)4[Pt2(pop)4Br2] |
2.716(1) |
2.572(1) |
Br |
59 |
||||||
K4[Pt2(pop)4Br2]·2H2O |
2.723(4) |
2.555(5) |
Br |
60 |
||||||
K4[Pt2(pop)4I2] |
|
|
2.754(1) |
2.746(1) |
I |
59 |
||||
K |
2 |
(Bun |
N) |
[Pt |
(pop) |
I ] |
2.742(1) |
2.721(1) |
I |
59 |
|
4 |
2 |
2 |
4 |
2 |
|
|
|
|
|
(Bun4N)2[Pt2(pop)4(SEt2)2] |
2.766(1) |
2.479(5) |
S |
62 |
||||||
K4[Pt2(pcp)4Cl2]·8H2O |
2.750(1) |
2.442(1) |
Cl |
51 |
||||||
K4[Pt2(pop)4(SCN)2]·2H2O |
2.760(1) |
2.466(4) |
S |
66 |
||||||
K4[Pt2(pop)4(NO2)2]·2KNO2·2H2O |
2.754(1) |
2.147(6) |
N |
66 |
||||||
K4[Pt2(pop)4(IM)2]·7H2O |
2.745(1) |
2.13(2) |
N |
66 |
||||||
(Bun4N)2[Pt2(pop)4(NCCH3)2] |
2.676(1) |
2.09(1) |
N |
67 |
||||||
Na8[Pt2(pop-H)4(NO2)2]·18H2O |
2.733(1) |
2.153(6) |
N |
65 |
||||||
[Pt2(O2CCH3)4(H2O)2](ClO4)2 |
2.391(1) |
2.17(1) |
O |
83 |
||||||
[Pt2(O2CCH3)4(H2O)2](CF3SO3)2·4H2O |
2.393(1) |
2.115(4) |
O |
83 |
||||||
Cs3[Pt2(O2CCH3)2(CH2CO2)2Cl2]Cl·3H2O (H,T) |
2.451(1) |
2.44[2] |
Cl |
92 |
||||||
Murillo |
|
Compounds Platinum and Palladium Nickel, |
|
637 |
|
Compounda
Pt2[S2CCH(CH3)2]4I2·I2
Pt2[S2CCH2Ph]4I2
Pt2[S2CC2H5]4I2
cis-Pt2(O2CCF3)2(CH3)4(4-Mepy)2
cis-Pt2(O2CCH3)2(CH3)4(py)2
Pt2(DPhF)4Cl2·THF
Pt2(hpp)4Cl2
Pt2(ButCONH)4Cl2·1.5H2O
Pt2(CH3CONH)4I2·8H2O
[Pt2(PPh3)2(ButCONH)4](NO3)2·2CHCl3
[Pt2(H2O)(PPh3)(ButCONH)4](NO3)2
cis-[Pt2(µ-CH3CONH)2(CH3CONH)2(en)2]I2 (H,T)
cis-[Pt2(ButCONH)2(NH3)4(NO2)(NO3)]·H2O (H,H)
cis-[Pt2(ButCONH)2(NH3)4(CH2COCH3)](NO3)3·H2O (H,H)
cis-[Pt2(ButCONH)2(NH3)4(CH2CH(CH2)3)O](NO3)3·7H2O (H,H)
cis-[Pt2(ButCONH)2(NH3)4(CH2CHO)](NO3)3·H2O (H,H)
cis-[Pt2(ButCONH)2(NH3)4(CH2COPh)](NO3)3·PhCOCH3 (H,H)
cis-[Pt2(ButCONH)2(NH3)4(CH2COCH2COCH3)](NO3)3 (H,H)
cis-[Pt2(ButCONH)2(NH3)4(CH2CHCH(OH)Me)](NO3)3 (H,H)
cis-[Pt2(ButCONH)2(NH3)4(CH2CHCH(OH)Et)](NO3)3 (H,H)
cis-[Pt2(ButCONH)2(NH3)4(CH2CMeCH(OH)Me)](NO3)3 (H,H)
cis-[Pt2(ButCONH)2(NH3)4(CH2CHCMe(OH)Me)](NO3)3 (H,H)
cis-[Pt2(ButCONH)2(NH3)4(CHC(OH)CH2CH2)](NO3)3 (H,H)
cis-[Pt2(ButCONH)2(NH3)4(CH2C(O)CH2CH2CH3)](NO3)3·H2O (H,H)
cis-[Pt2(ButCONH)2(NH3)4(CH2C(O)CH2OH](NO3)3·0.5C3H5O (H,H)
r(Pt–Pt) (Å) |
r(Pt–L) (Å)b |
Donor |
ref. |
|
atom(s) L |
||||
|
|
|
||
2.578(1) |
2.764[2] |
I |
94 |
|
2.598(2) |
2.753(3) |
I |
94 |
|
2.582(2) |
2.764[1] |
I |
95 |
|
2.557(1) |
2.13[4] |
N |
99 |
|
2.529(1) |
2.20(1) |
N |
100 |
|
2.517(1) |
2.435(2) |
Cl |
86 |
|
2.438(1) |
2.483(4) |
Cl |
101 |
|
2.448(2) |
2.427(5) |
Cl |
110 |
|
2.473(2) |
2.733(3) |
I |
109 |
|
2.504(1) |
2.460(4) |
P |
112 |
|
2.468(1) |
2.404(3) |
P |
112 |
|
|
2.279(6) |
O |
|
|
2.567(1) |
2.16(2) |
N |
113 |
|
2.609(1) |
2.29(1) |
O(NO3-) |
114 |
|
|
2.10(1) |
N(NO -) |
|
|
|
|
2 |
|
|
2.689(1) |
2.095(9) |
C |
114 |
|
|
|
|
|
|
|
2.667(7) |
O |
|
|
2.711(1) |
2.11 |
C |
119 |
|
2.749(1) |
2.121(9) |
C |
119 |
|
2.676(1) |
2.15(2) |
C |
120 |
|
2.721(1) |
2.114(8 |
C |
120 |
|
2.734(1) |
2.12(1) |
C |
122 |
|
2.735(1) |
2.06(1) |
C |
122 |
|
2. 740(1) |
2.14(1) |
C |
122 |
|
2.732(1) |
2.11(1) |
C |
122 |
|
2.734(2) |
2.11(3) |
C |
121 |
|
2.688(1) |
2.10(2) |
C |
123 |
|
2.700(1) |
2.10(1) |
C |
123 |
|
|
|
|
|
|
638 |
|
14 Chapter |
|
Bonds Multiple |
|
||
|
|
Atoms Metal Between |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Compounda |
r(Pt–Pt) (Å) |
r(Pt–L) (Å)b |
Donor |
ref. |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
atom(s) L |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
cis-[Pt2(ButCONH)2(NH3)4(CH2C(O)(CH2)3OH](NO3)3 (H,H) |
2.688(1) |
2.10(2) |
C |
|
123 |
||||||||||||||||||||||
cis-[Pt2(ButCONH)2(NH3)4(CH2C(O)CH2OCH3](NO3)3 (H,H) |
2.693(1) |
2.09(1) |
C |
|
123 |
||||||||||||||||||||||
cis-[Pt |
2 |
(ButCONH) |
2 |
(NH |
3 |
) |
4 |
(CH |
2 |
(CH )C(O)CH |
](NO |
) |
3 |
(H,H) |
2.722 |
2.15(1) |
C |
|
123 |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
3 |
3 |
3 |
|
|
|
|
|
|
|
||||||
cis-[Pt2(hp)2(NH3)4(NO3)(H2O)](NO3)3·2H2O (H,H) |
|
|
|
2.540(1) |
2.193(7) |
O(NO3-) |
132 |
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.122(6) |
O |
|
|
cis-[Pt2(hp)2(NH3)4(NO3)2](NO3)2·0.5H2O (H,T) |
|
|
|
2.547(1) |
2.17(1) |
O(NO3-) |
132 |
||||||||||||||||||||
cis-[Pt2(hp)2(NH3)4(NO2)2](NO3)2·0.5H2O (H,T) |
|
|
|
2.576(1) |
2.170[2] |
N(NO2-) |
133 |
||||||||||||||||||||
cis-[Pt2(hp)2(NH3)4Cl2](NO3)2 (H,T) |
|
|
|
|
|
|
2.568(1) |
2.436[8] |
Cl |
|
133 |
||||||||||||||||
cis-[Pt2(hp)2(NH3)4Br2](NO3)2·0.5H2O (H,T) |
|
|
|
|
2.582(1) |
2.568[6] |
Br |
|
133 |
||||||||||||||||||
cis-[Pt |
2 |
(hp) |
2 |
(en) |
(NO |
)(NO |
3 |
)](NO |
) |
·0.5H O (H,H) |
|
|
|
2.638(1) |
2.307(9) |
O(NO |
-) |
134 |
|||||||||
|
|
2 |
|
|
2 |
|
|
|
|
|
|
3 |
2 |
|
2 |
|
|
|
|
|
|
|
3 |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.11(1) |
N |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
cis-[Pt2(1-MeC)2(NH3)4(NO2)2](NO3)2·2H2O (H,T) |
|
|
|
2.584(1) |
2.12[2] |
N |
|
135,136 |
|||||||||||||||||||
cis-[Pt2(1-MeC)2(NH3)2(gly-N,O)2](NO3)2·3H2O (H,T) |
|
2.527(1) |
2.17[1] |
N |
|
137 |
|||||||||||||||||||||
cis-[Pt2(l-MeC)2(NH3)4(NO3)2](NO3)2·HNO3·3H2O (H,T) |
2.552(1) |
2.138(8) |
O |
|
138 |
||||||||||||||||||||||
cis-[Pt2(l-MeC)2(NH3)4(NO2)(H2O)](ClO4)3·3.5H2O (H,T) |
2.604(1) |
2.091(8) |
N |
|
138 |
||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.311(6) |
O |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
cis-[Pt2(l-MeC)2(NH3)4(H2O)2](ClO4)4·H2O (H,T) |
|
|
|
2.565(1) |
2.16[4] |
O |
|
138 |
|||||||||||||||||||
cis-[Pt2(l-MeC)2(NH3)4(EtguaH)2](NO3)4·9H2O (H,T) |
|
|
|
2.586(1) |
2.184[7] |
N |
|
138,139 |
|||||||||||||||||||
cis-Pt2(hp)2(CH3)4(py)2·2CHCl3 (H,T) |
|
|
|
|
|
2.550(1) |
2.18[2] |
N |
|
100 |
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
cis-Pt2(hp)2(CH3)4(py) (H,H) |
|
|
|
|
|
|
|
|
|
2.556(1) |
2.034(8) |
N |
|
127 |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
cis-Pt2(fhp)2(CH3)4(py)2·0.17C6H6 (H,T) |
|
|
|
|
|
2.551(1) |
2.20[1] |
N |
|
100 |
|||||||||||||||||
cis-Pt2(fhp)2(CH3)4(py) (H,H) |
|
|
|
|
|
|
|
|
|
2.554(1) |
2.04(1) |
N |
|
127 |
|||||||||||||
cis-Pt2(chp)2(CH3)4(py) (H,H) |
|
|
|
|
|
|
|
|
|
2.543(1) |
2.06(1) |
N |
|
100 |
|||||||||||||
cis-Pt2(mhp)2(CH3)4(py) (H,H) |
|
|
|
|
|
|
|
|
|
2.545(1) |
2.030(8) |
N |
|
100 |
|||||||||||||
cis-Pt2(bhp)2(CH3)4(py) (H,H) |
|
|
|
|
|
|
|
|
|
2.551(1) |
2.06(2) |
N |
|
127 |
|||||||||||||
cis-Pt2(hp)2(CH3)4(SEt2)·0.5C7H8 (H,H) |
|
|
|
|
|
2.568(1) |
2.292(4) |
S |
|
128 |
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
cis-Pt2(fhp)2(CH3)4(SEt2)·0.5C7H8 (H,H) |
|
|
|
|
|
2.571(1) |
2.285(4) |
S |
|
128 |
|||||||||||||||||
cis-Pt2(mhp)2(CH3)4(SEt2) (H,H) |
|
|
|
|
|
|
|
|
2.561(1) |
2.303(4) |
S |
|
128 |
||||||||||||||
Murillo |
|
Compounds Platinum and Palladium Nickel, |
|
639 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Compounda |
r(Pt–Pt) (Å) |
r(Pt–L) (Å)b |
Donor |
ref. |
|
|
|
|
|
|
|
|
|
|
|
|
|
atom(s) L |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
cis-[Pt2(1-MeU)2(NH3)4(NO2)(H2O)](NO3)3·5H2O (H,T) |
2.574(1) |
2.08(1) |
N |
142 |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.253(9) |
O |
|
|
|
|
|
|
|||||||||||||
cis-[Pt2(1-MeU)2(NH3)4(NO3)(H2O)](NO3)3·3H2O (H,T) |
2.556(1) |
2.14(1) |
O(NO3-) |
143 |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.18(1) |
O |
|
|
|
|
|
|
|||||||||||||
cis-[Pt2(1-MeU)2(NH3)4(NO3)(H2O)](NO3)3·2H2O (H,T) |
2.560(1) |
2.12(1) |
O(NO3-) |
143 |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.17(1) |
O |
|
cis-[Pt2(1-MeU)2(NH3)4(NO2)](NO3)3·H2O (H,H) |
2.607(1) |
2.06(2) |
N |
144 |
|||||||||||||
cis-[Pt2(1-MeU)2(NH3)4Cl2]Cl2·3.5H2O (H,H) |
2.573(1) |
2.44[2] |
Cl |
145 |
|||||||||||||
cis-Pt2(1-MeU)2(NH3)2Cl4·2H2O (H,H) |
|
|
2.543(1) |
2.44[2] |
Cl |
145 |
|||||||||||
cis-[Pt2(1-MeU)2(NH3)4(1-MeU)](SiF6)(NO3)·7H2O (H,H) |
2.685(1) |
2.037(9) |
C |
146 |
|||||||||||||
cis-[Pt |
2 |
(pyrr) |
2 |
(NH |
3 |
) |
4 |
(NO )(NO |
)](NO |
) |
·H |
O (H,H) |
2.644(1) |
2.01[1] |
N(NO -) |
148 |
|
|
|
|
|
2 |
3 |
3 |
2 |
2 |
|
|
|
2 |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.00[1] |
O(NO3-) |
|
cis-[Pt2(pyrr)2(NH3)4Cl2](NO3)2 (H,H) |
|
|
|
2.637(1) |
2.395(3) |
Cl |
150 |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.455(3) |
Cl |
|
|
|
|
|
|
|||||||||||||
cis-[Pt2(pyrr)2(NH3)4Cl(NO3)](NO3)2·H2O (H,H) |
2.624(1) |
2.361(4) |
Cl |
150 |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.30(3) |
O |
|
|
|
|
|
|
|||||||||||||
cis-[Pt2(pyrr)2(NH3)3(H2O)(µ-OH)]2(NO3)6·4H2O (H,T) |
2.553(1) |
2.188(6) |
O(H2O) |
150 |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NA |
O(OH) |
|
cis-[Pt2(pyrr)2(NH3)4(C10H13N5O4)(SO4)]·4H2O (H,H) |
2.584(1) |
2.13(1) |
N |
152 |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.199(8) |
O |
|
|
|
|
|
|
|||||||||||||
cis-[Pt2(pyrr)2(NH3)3(µ-NH2)(NO3)]2(NO3)4·4H2O (H,H) |
2.608(1) |
1.98(1) |
N |
151 |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.29(1) |
O |
|
|
|
|
|
|
|
||||||||||||
cis-[Pt2(1-MeT)2(NH3)4(NO2)](NO3)3 (H,H) |
|
2.651(1) |
2.079(7) |
N |
155 |
||||||||||||
cis-[Pt2(1-MeT)2(NH2CH3)2Cl3]ClO4 (H,H) |
|
2.612(2) |
2.31(1) |
Cl |
155 |
||||||||||||
cis-[Pt2(1-MeT)2(NH3)2Cl3(H2O)](PtCl6)0.5·H2O·0.4HCl |
2.556(1) |
d |
|
140 |
|||||||||||||
cis-2,2-[Pt2(1-MeC)4(NH3)2](ClO4)2·2H2O |
|
2.465(1) |
2.178[7] |
N |
156 |
||||||||||||
cis-2,2-[Pt2(1-MeC)4(NH3)(H2O)](ClO4)2·1.6H2O |
2.451(1) |
2.112(7) |
N |
156 |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.259(6) |
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
640 |
|
14 Chapter |
|
Bonds Multiple |
|
||
|
|
Atoms Metal Between |
|
|
|
|
|
|
|
|
|
Compounda |
r(Pt–Pt) (Å) |
r(Pt–L) (Å)b |
Donor |
ref. |
|
|
|
|
|
|
|
|
|
atom(s) L |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
cis-2,2-[Pt2(1-MeC)4(NO2)]ClO4·6H2O |
2.498(1) |
2.044(1) |
N |
156 |
|||||||||
Pt2(pymS)4Cl2 |
|
|
|
|
|
|
2.518(1) |
2.45[1] |
Cl |
158 |
|||
Pt2(pymS)4I2 |
|
|
|
|
|
|
2.554(1) |
2.774[6] |
I |
157 |
|||
Pt2(2-TU)4I2 |
|
|
|
|
|
|
2.546(2) |
2.771[5] |
I |
158 |
|||
Pt2(pyS)4Cl2·2CHCl3 |
|
|
|
|
|
2.532(1) |
2.458(2) |
Cl |
159 |
||||
[Pt2(5-MepyS)4Cl]S4[Pt2(5-MepyS)4Cl]·0.5S8·2CHCl3 |
2.556(2) |
2.603(7) |
Cl |
161 |
|||||||||
|
|
|
|
|
|
|
|
|
|
2.558(2) |
2.567(8) |
Cl |
|
|
|
|
|
|
|
|
|
|
|
|
2.435(8) |
S |
|
|
|
|
|
|
|
|
|
|
|
|
2.424(9) |
S |
|
|
|
|
|
|
|||||||||
[Pt2(5-MepyS)4Br]S4[Pt2(5-MepyS)4Br]·0.5S8·2CHCl3 |
2.560(2) |
2.705(5) |
Br |
161 |
|||||||||
|
|
|
|
|
|
|
|
|
|
2.552(2) |
2.660(6) |
Br |
|
|
|
|
|
|
|
|
|
|
|
|
2.42(1) |
S |
|
|
|
|
|
|
|
|
|
|
|
|
2.41(1) |
S |
|
|
|
|
|
|
|
|
|
||||||
cis-Pt2Cl6[HN=C(OH)CMe3]4e |
|
|
|
2.694(1) |
2.458(3) |
Cl |
163 |
||||||
cis-Pt2Cl6[(E)HN=C(OMe)Me]4·C6H6e |
|
2.765(2) |
2.474(7) |
Cl |
164 |
||||||||
|
|
|
|
|
|
|
|
|
|
|
2.465(7) |
Cl |
|
|
|
|
|
|
|
|
|
|
|||||
trans-Pt |
Cl [(E)HN=C(OH)CMe |
] |
·C |
H e |
2.758(3) |
2.450(6) |
Cl |
164 |
|||||
|
|
2 |
6 |
|
|
3 |
4 |
6 |
6 |
|
|
|
|
Pt2(C8H12(=NO)H)4Cl2e |
|
|
|
|
2.696(1) |
2.40[1] |
Cl |
166 |
|||||
Pt2(phpy)4Cl2e |
|
|
|
|
|
|
2.727(1) |
2.16[1] |
N |
165 |
|||
Pt |
2 |
(OBQDI-H) |
(CF |
SO ) e |
|
|
|
3.031(1) |
|
|
167 |
||
|
|
4 |
3 |
|
3 2 |
|
|
|
|
|
|
|
|
a In those cases where a head-to-tail or head-to-head arrangement of cis bridging ligands is possible, the actual isomer characterized is denoted by (H,T) or (H,H).
b In some cases the average Pt–L lengths are quoted. In these instances the estimated deviation, which is given in square brackets, is calculated as [ ] = [−n¨i2/n(n-1)]1/2, in which
¨i is the deviation of the ith of n values from the arithmetic mean of the set.
c Average value for two crystallographically independent molecules in the unit cell. d Not reported.
e This complex contains an unsupported Pt–Pt bond.
Murillo |
Compounds Platinum and Palladium Nickel, |
641

642Multiple Bonds Between Metal Atoms Chapter 14
14.4.1 Complexes with sulfate and phosphate bridges
The first entirely unequivocal identification of a diplatinum(III) complex was the tetra- kis-µ-sulfato derivative K2[Pt2(SO4)4(H2O)2], whose crystal structure was determined but only incompletely reported in 1976.29 The anion has the same type of sulfato-bridged structure, with two axial water molecules, as that of the [Re2(SO4)4(H2O)2]2- ion and several other similar ones. The Pt–Pt distance of 2.466 Å (quoted without an esd) is consistent with the assumption that a single bond, based on a μ2/4β2β*2/*4 configuration, exists between the PtIII atoms. The dinuclear anion is formed in a complex reaction,29,30 which is said to have the following overall stoichiometry:
2Pt(NO2)2(NH3)2 |
H2SO4 |
(NH4)2[Pt2(SO4)4(H2O)2] + 2NO + 2NH4HSO4 |
|
This compound has also been prepared by using K2[Pt(NO2)4] in place of Pt(NO2)2(NH3)2.31 A subsequent redetermination of this structure gave32 a Pt–Pt bond distance of 2.461(1) Å, essentially identical to the previously reported29 value. The lability of the axial water molecules is shown33 by their ease of displacement by dimethylsulfoxide to form K2[Pt2(SO4)4(DMSO)2]. In this complex the DMSO ligands are O-bound, and the Pt–Pt distance is a little longer (by c. 0.01 Å) than in the aquo adduct.33 From boiling pyridine, yellow crystals of (pyH)2[Pt2(SO4)4(py)2] can be isolated.34 This complex, shown in Fig. 14.3, reacts with a 50% solution of boiling acetic acid for 20 h displacing only two of the four bridging sulfate groups by acetate anions. The axial water molecules in K2[Pt2(SO4)4(H2O)2] can be easily replaced also by heating with pyridazine in aqueous solution but partial reduction also occurs. This gives [Pt(pydz)4][Pt2(SO4)4(pydz)2].35 Further heating cleaves the Pt–Pt bond giving compounds with the [Pt(pydz)4]2+ ion exclusively. Other derivatives with various neutral and monoanionic axial ligands (e.g., NH3, NH2CH3, ROH, Cl-, Br-, CN-, NO2-, SCN- or OH-) have also been described.30,31,36,38 Measurements have been made of the 195Pt NMR spectra of several of these complexes.31,37 Of special interest is the chiral complex K2[Pt2(SO4)4(Amb)2], where Amb is the optically active R(-)-2-amino-1-butanol. This adduct has been studied by circular dichroism, IR, XPS and NMR spectroscopies.38
Fig. 14.3. The structure of the [Pt2(SO4)4(py)2]2- anion.
Several phosphato-bridged diplatinum(III) complexes have also been prepared and structurally characterized. Most of these have been shown to contain the dianionic monohydrogenphosphate ligand [HPO4]2-. The synthesis of a complex purported to be (NH4)2{(H)4-
[Pt2(PO4)4(H2O)2]}, as well as several of its derivatives in which the H2O molecules are
