
Multiple Bonds Between Metal Atoms / 04-Molybdenum Compounds
.pdf
4
Molybdenum Compounds
F. Albert Cotton,
Texas A&M University
4.1Dimolybdenum Bridged by Carboxylates or Other O,O Ligands
4.1.1 General remarks
There are more M2n+ compounds with multiply-bonded M2n+ units having M = Mo than any other metal (although Rh24+ compounds (Chapter 12), in which there is a single bond, are more numerous). The total number of Mo2n+ compounds, is over 1100, of which about 550 have been crystallographically characterized.
The somewhat muddled early preparative work on Mo2(O2CR)4 compounds has been reviewed in Chapter 1. Productive development of the field followed closely on the structural characterization of Mo2(O2CCH3)4.1 This structure as later redetermined more precisely,2 is shown in Fig. 4.1; the Mo–Mo distance is 2.093(1) Å, a typical value for Mo–Mo quadruple bonds.
Fig. 4.1. The structure of Mo2(O2CCH3)4 as first accurately reported.
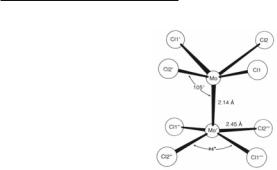
The conversion of Mo2(O2CCH3)4 to Mo2Cl84−, and the definitive structural characterization of this anion, Fig. 4.2, were reported in 1969.3 The observation that the Mo2Cl84− anion is stereoelectronically analogous to Re2Cl82− was a critical breakthrough in the evolution of Mo24+ chemistry.
69
70Multiple Bonds Between Metal Atoms Chapter 4
Fig. 4.2. The structure of the Mo2Cl84− ion in K4Mo2Cl8·H2O, exactly as first reported in 1969.
4.1.2 Mo2(O2CR)4 compounds
Preparation of Mo2(O2CR)4 compounds.
Synthesis of the carboxylates from a mononuclear starting material, molybdenum hexacarbonyl, was first described by Wilkinson and co-workers. The procedure used by Wilkinson,4-6 namely, heating Mo(CO)6 with the carboxylic acid (and the anhydride if available) either alone or in diglyme, still remains a good general method for preparing these complexes7-10 although modifications of this procedure have sometimes been used. Such modifications include the use of solvents other than diglyme (for example, decalin, 1,2-dichlorobenzene, and toluene),8,11 a different carbonyl precursor (for example, Mo(CO)4[(CH3)2NCH2CH2N(CH3)2]),11 and carboxylate exchange reactions utilizing the acetate Mo2(O2CCH3)4 as the starting material.8,12-19 While other methods have been reported for the synthesis of the carboxylates, these usually involve starting materials that are themselves first prepared from Mo2(O2CCH3)4 (e.g. salts of the [Mo2Cl8]4− anion). However, one unusual exception is the preparation of the formate Mo2(O2CH)4 from [Mo(δ6-C6H5Me)(δ3-C3H5)Cl]2.20 Other examples are the reaction of MoCl3(THF)3 with Zn and acetic acid to give Mo2(O2CCH3)4,21 and the reduction of MoCl3 by Na/Hg in the presence of NaO2CCF3 to produce very pure Mo2(O2CCF3)4.22 As far as the range of carboxylate ligands that have been used to form neutral complexes of the type Mo2(O2CR)4 is concerned, these have included formic acid,13 several alkyl,3,19a halo-alkyl,3,8,12 and aryl monocarboxylic acids,3,9 as well as dicarboxylic acids14 and mixed monoand di-carboxylates.19b,c Another interesting and important group of complexes are those in which chiral ligands are used, e.g., mandelic acid.23 Deuterated derivatives can also be prepared, for example Mo2(O2CCD3)4, a compound whose vibrational spectral properties have been of interest.24
While the formation of Mo2(O2CCH3)4 from Mo(CO)6 is the single most important synthesis of a dimolybdenum(II) carboxylate, this reaction proceeds in low yield (15-20%) when pure acetic acid or a mixture of the acid and its anhydride is used.10 Superior yields (80%) are obtained only when a solvent such as diglyme or 1,2-dichlorobenzene (mixed with a small amount of hexane) is used. The fate of the remaining molybdenum has been established in a variety of subsequent studies.25 It is converted to one or more higher oxidation state trinuclear species of the type [Mo3X2(O2CCH3)6(H2O)3]n+ where the Mo3X2 unit is a trigonal bipyramid in which the axial or capping units, X, are either O or CCH3, or one of each. There are Mo–Mo single bonds in these clusters.
In addition to Mo2(O2CCH3)4, the structure of which is shown in Fig. 4.1, a number of other structures of Mo2(O2CR)4 compounds have been reported. All of these are listed in Table 4.1. The structure of the acetate is prototypical for most of them; in this type of structure there

Molybdenum Compounds 71
Cotton
are no exogenous axial ligands and the Mo2(O2CR)4 molecules are strung together in infinite chains, very similar to the chains found in Cr2(O2CCH3)4, shown in Fig. 3.1b. In all these cases, the Mo–Mo quadruple bond lengths are about the same, c. 2.10 Å. The intermolecular Mo···O links are quite long (2.60-2.90 Å) compared to the intramolecular Mo–O bonds (c. 2.10 Å).
Table 4.1. Structures of Mo2(O2CR)4 compounds |
|
|
|
|
|||||||||||||||||||
|
|
|
|
Compound |
|
|
|
|
|
|
Crystal |
Virtual |
Mo–Mo |
Twista |
ref. |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sym. |
Sym. |
|
|
|
Mo2(O2CH)4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
D4h |
2.091(2) |
1.0 |
13 |
||||
Mo2(O2CH)4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
D4h |
2.093(1) |
0 |
35 |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
D4h |
2.092(1) |
50 |
35 |
Mo2(O2CH)4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
D4h |
2.091(1) |
0 |
35 |
||||
Mo2(O2CH)4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
D4h |
2.091(1) |
0 |
28 |
||||
Mo2(O2CH)4(H2O)2 |
|
|
|
|
|
|
|
|
|
|
|
|
1 |
D4h |
2.100(1) |
0 |
28 |
||||||
Mo2(O2CH)4·KCl |
|
|
|
|
|
|
|
|
|
|
|
|
1 |
D4h |
2.109(2) |
0 |
35 |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
D4h |
2.102(2) |
0 |
35 |
Mo2(O2CCH3)4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
D4h |
2.093(1) |
0 |
2 |
|||||
Mo2(O2CCH3)4·NaO2CCH3·HO2CCH3 |
1 |
D4h |
2.093(1) |
0 |
33 |
||||||||||||||||||
Mo2(O2CCH3)4(µ-dmpe) |
|
|
|
|
|
|
|
|
1 |
D4h |
2.105(3) |
0 |
49 |
||||||||||
Mo2(O2CCH3)4(µ-tmed) |
|
|
|
|
|
|
|
|
1 |
D4h |
2.103(1) |
0 |
49 |
||||||||||
[ |
|
|
|
|
|
|
|
|
|
|
|
|
] |
|
|
|
|
|
¯ |
C2v |
2.124(2) |
NR |
50 |
|
Mo2(O2CCH3)4(N,N'-dmed) |
|
|
|
|
1 |
|||||||||||||||||
dmed = Me(H)NCH2CH2N(H)Me |
|
|
|
|
|
|
|
|
|
||||||||||||||
[Mo2(O2CCH3)4(N,N'-pda)] |
|
|
|
|
|
1 |
C2v |
2.130(2) |
NR |
50 |
|||||||||||||
pda = H2NCH2CH2CH2NH2 |
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Mo2(O2CCH3)4(µ-4,4'-bipyridine)·THF |
1 |
C2h |
2.103(1) |
0 |
51 |
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C2h |
2.104(1) |
0 |
51 |
Mo2(O2CCH3)4(4,4'-bipyr) |
] |
n·nTHF |
|
|
|
|
¯ |
D4h |
2.104(1) |
zero |
52 |
||||||||||||
|
|
|
|
|
1 |
||||||||||||||||||
(pyH)2[Mo2(O2CCH3)4)Br2] |
|
|
|
|
|
|
4/m |
D4h |
2.101(1) |
0 |
53 |
||||||||||||
(pyH)2[Mo2(O2CCH3)4)I2] |
|
|
|
|
|
|
|
1 |
D4h |
2.103(1) |
0 |
53 |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
D4h |
2.103(1) |
0 |
53 |
(pyH)2[Mo2(O2CCH3)4)I2] |
|
|
|
|
|
|
|
4/mmm |
D4h |
2.102(1) |
0 |
53 |
|||||||||||
(PipH)2 |
[ |
Mo2(O2CCH3)4I2 |
] |
|
|
|
|
|
|
|
¯ |
D4h |
2.100(3) |
zero |
54 |
||||||||
|
|
|
|
|
|
|
|
|
|
1 |
|||||||||||||
Mo2(O2CCF3)4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
D4h |
2.090(4) |
0 |
12 |
|||||
Mo2(O2CCF3)4(py)2 |
|
|
|
|
|
|
|
|
|
|
|
|
1 |
D4h |
2.129(2) |
0 |
29 |
||||||
Mo2(δ1-O2CCF3)4(bpy)2 |
|
|
|
|
|
|
|
|
|
1 |
C2h |
2.129(1)b |
0 |
55 |
|||||||||
Mo2(O2CCF3)4(PBun3)2 |
|
|
|
|
|
|
|
|
|
|
1 |
C2h |
2.105(1) |
0 |
22 |
||||||||
Mo2(O2CCF3)4(PEt2Ph)2 |
|
|
|
|
|
|
|
|
|
1 |
C2h |
2.100(1) |
0 |
56 |
|||||||||
Mo2(O2CCF3)4(PMePh2)2 |
|
|
|
|
|
|
|
|
1 |
D4h |
2.128(1) |
50 |
57 |
||||||||||
Mo2(O2CCF3)4(PMePh2)2 |
|
|
|
|
|
|
|
|
1 |
C2h |
2.107(2) |
0 |
56 |
||||||||||
(Bu4N)2[Mo2(O2CCF3)4Br2] |
|
|
|
|
|
|
1 |
D4h |
2.134(2) |
0 |
58 |
||||||||||||
(Bu4N)2[Mo2(O2CCF3)4I2] |
|
|
|
|
|
|
|
1 |
D4h |
2.140(2) |
0 |
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
D4h |
2.136(2) |
0 |
|
[ |
Mo2(O2CCF3)4(DM-DCNQI)·C6H6 |
] |
|
|
¯ |
D4h |
2.127(2) |
zero |
59 |
||||||||||||||
|
|
n |
1 |
||||||||||||||||||||
[ |
Mo2(O2CCF3)4(TCNQ)0.5·m- |
|
|
|
|
|
¯ |
D4h |
2.113(1) |
NR |
60 |
||||||||||||
|
|
|
|
|
|
1 (chain) |
|||||||||||||||||
xylene] |
|
·[Mo |
(O |
CCF |
3 |
) |
·m-xylene] |
|
|
|
|
¯ |
D4h |
2.113(1) |
zero |
|
|||||||
|
2 |
|
2 |
2 |
|
|
4 |
|
|
|
|
|
|
|
|
|
1 (molec.) |
|
|||||
Mo2(O2CCF3)4·9,10-anthraquinone |
] |
|
|
|
¯ |
D4h |
2.107(1) |
NR |
61 |
||||||||||||||
|
n |
|
|
1 |
|||||||||||||||||||
[ |
Mo2(O2CCF3)4·bpy |
] [ |
|
|
|
|
|
|
|
|
] |
· |
¯ |
D4h |
2.124(1) |
zero |
62 |
||||||
|
n |
Mo2(O2CCF3)4 |
|
Dimer: 1 |
|||||||||||||||||||
(bpy)2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chain: 1 |
D4h |
2.128(1) |
NR |
|
|

72Multiple Bonds Between Metal Atoms Chapter 4
|
Compound |
|
|
Crystal |
Virtual |
Mo–Mo |
Twista |
ref. |
|||||
|
|
|
|
|
|
|
|
|
Sym. |
Sym. |
|
|
|
Mo2(O2CCF3)4(FPA)2 |
|
|
|
|
|
¯ |
D4h |
2.142(1) |
zero |
63 |
|||
|
|
|
|
|
1 |
||||||||
FPA = (ferrocenyl)(4-pyridyl)ethyne |
|
|
|
|
|
|
|
||||||
Mo2(O2CCF3)4( |
δ1 |
-HO2CCF3)3·2Hdpa |
|
|
¯ |
D4h |
2.131(1) |
zero |
64 |
||||
|
|
|
1 |
||||||||||
Mo2(O2CCF3)4(1,4-nq) |
] |
n·nC6H6 |
|
|
¯ |
D4h |
2.117(1) |
zero |
65 |
||||
|
|
|
1 |
||||||||||
nq = naphthoquinone |
|
|
|
|
|
|
|
|
|
||||
Mo2(O2CCF3)4-bis(2,6-di-t-butyl-p-ben- |
¯ |
D4h |
2.114(1) |
zero |
66 |
||||||||
1 |
|||||||||||||
zoquinone) |
|
|
|
|
|
|
|
|
|
|
|
|
|
_-Mo2(O2CCMe3)4 |
|
|
|
|
|
1 |
D4h |
2.088(1) |
50 |
37 |
|||
`-Mo2(O2CCMe3)4 |
|
|
|
|
|
1 |
D4h |
2.087(1) |
0 |
38 |
|||
α-Mo2(O2CCMe3)4 |
|
|
|
|
|
1 |
D4h |
2.087(1) |
0 |
38 |
|||
[ |
Mo2(O2CCMe3)4·bpy |
] |
n |
|
|
|
¯ |
D4h |
2.092(1) |
zero |
67 |
||
|
|
|
|
|
1 |
||||||||
|
|
|
|
|
|
|
|
|
|
|
2.099(1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Mo2(O2CC6H5)4 |
|
|
|
|
|
|
1 |
D4h |
2.096(1) |
0 |
37 |
||
Mo2(O2Cadamantyl)4·2THF |
|
|
¯ |
D4h |
2.087(1) |
zero |
68 |
||||||
|
|
1 |
|||||||||||
|
|
|
|
|
|
|
|
|
¯ |
D4h |
2.087(1) |
zero |
|
|
|
|
|
|
|
|
|
|
1 |
|
|||
Mo2(O2CC6H5)4(diglyme)2 |
|
|
1 |
D4h |
2.100(1) |
0 |
69 |
||||||
Mo2(O2CC6H4-2-Ph)4 |
|
|
|
|
1 |
D4h |
2.082(1) |
0 |
15 |
||||
(Ph4P)2[Mo2(O2CC6H5)4Cl2]·2CH2Cl2 |
|
|
1 |
D4h |
2.128(1) |
0 |
70 |
||||||
(Ph4P)2[Mo2(O2CC6H5)4Br2]·2CH2Br2 |
|
|
1 |
D4h |
2.123(1) |
0 |
71 |
||||||
Mo2(µ-O2CC6H3(NH3)2)4Cl8·16H2O |
|
|
¯ |
D4h |
2.107(1) |
zero |
72 |
||||||
|
|
1 |
|||||||||||
Mo2(O2CC6H4-3-NO2)4(py)2·2py |
|
|
¯ |
D4h |
2.125(1) |
zero |
50 |
||||||
|
|
1 |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
2.123(1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Mo2(O2CC6H4Ph)4 |
|
|
|
|
|
1 |
D4h |
2.096(1) |
NR |
73 |
|||
Mo2(O2CC6H4Ph)4py2 |
|
|
|
|
¯ |
D4h |
2.111(1) |
zero |
73 |
||||
|
|
|
|
1 |
|||||||||
Mo2(µ-O2CC6H4-2-PPh2)4(MeOH)2 |
|
|
¯ |
D4h |
2.112(1) |
zero |
74 |
||||||
|
|
1 |
|||||||||||
[ |
Mo2(µ-O2CC6H4-4-P(O)Ph2)4·4EtOH |
] |
n |
¯ |
D4h |
2.125(2) |
zero |
74 |
|||||
|
|
1 |
|||||||||||
Mo2(O2C-o-C6H4Cl)4·4THF |
|
|
¯ |
D4h |
2.103(1) |
zero |
75 |
||||||
|
|
1 |
|||||||||||
Mo2(O2C-o-C6H4Br)4·2THF |
|
|
¯ |
D4h |
2.101(1) |
zero |
75 |
||||||
|
|
1 |
|||||||||||
Mo2(O2C-o-C6H4I)4·2THF |
|
|
¯ |
D4h |
2.106(1) |
zero |
75 |
||||||
|
|
1 |
|||||||||||
Mo2(O2C-o-C6H4NO2)4 |
|
|
|
¯ |
D4h |
2.094(1) |
zero |
75 |
|||||
|
|
|
1 |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
2.096(1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mo2(TiPB)4 |
|
|
|
|
|
|
|
¯ |
D4h |
2.076(1) |
zero |
27 |
|
|
|
|
|
|
|
|
1 |
||||||
TiPB = 2,4,6-triisopropylbenzoate |
|
|
|
|
|
|
|
||||||
Mo2(salicylate)4·1,2-C6H4Cl6 |
|
|
1 |
C2h |
2.092(1) |
0 |
76 |
||||||
|
|
|
|
|
|
|
|
|
1 |
C2h |
2.094(1) |
50 |
76 |
Mo2(salicylate)4(diglyme)2 |
|
|
1 |
C2h |
2.101(1) |
0 |
76 |
||||||
Mo2(D-mandelate)4·2THF |
|
|
1 |
C4 |
2.104(1) |
50 |
23 |
||||||
|
|
|
|
|
|
|
|
|
1 |
C4 |
2.101(1) |
50 |
|
Mo2(O2CC4H3S)4(THF)2 |
|
|
1 |
C2h |
2.102(1) |
0 |
77 |
||||||
Mo2(FCA)4(NCCH3)(DMSO)·2DMSO |
|
|
1 |
D4h |
2.105(1) |
0 |
16 |
||||||
Mo2(O2CCH3)2(FCA)2(py)2 |
|
|
1 |
C2h |
2.107(2) |
0 |
16 |
||||||
Mo2(O2CPBut2)4·2C6H6 |
|
|
|
1 |
D4h |
2.092(3) |
0 |
78,79 |
|||||
[Mo2(O2CCH2NH3)4](SO4)2·4H2O |
|
|
4 |
D4h |
2.115(1) |
0 |
80 |
||||||
[Mo2(O2CCH2NH3)4]Cl4·3H2O |
|
|
1 |
D4h |
2.112(1) |
0 |
81 |
||||||
[Mo2(O2CCH2NH3)4]Cl4·22/3H2O |
|
|
1 |
D4h |
2.103(1) |
0 |
81 |
||||||
|
|
|
|
|
|
|
|
|
1 |
D4h |
2.107(1) |
0 |
|
|
|
|
|
|
|
|
|
|
1 |
D4h |
2.110(1) |
0 |
|

Molybdenum Compounds 73
|
|
|
|
|
Cotton |
Compound |
Crystal |
Virtual |
Mo–Mo |
Twista |
ref. |
|
Sym. |
Sym. |
|
|
|
[Mo2(glygly)4]Cl4·6H2O |
1 |
D2h |
2.106(1) |
0 |
82 |
[Mo2(L-leu)4]Cl2(PTS)2·2H2O |
1 |
C4h |
2.108(1) |
50 |
83 |
|
1 |
C4h |
2.111(1) |
50 |
|
[Mo2(D-phe)2(L-phe)2I2]I2·6H2O |
1 |
D4h |
2.114(1) |
0 |
84 |
[Mo2(D-tyr)2(L-tyr) 2I2]I2·6H2O |
1 |
D4h |
2.116(1) |
0 |
84 |
[Mo2(D-phgly)2(L-phgly)2](PTS)4·4H2O |
1 |
D4h |
2.113(1) |
0 |
84 |
[Mo2(D-val)2(L-val)2](ZnCl4)2·4H2O |
1 |
D4h |
2.104(1) |
0 |
85 |
[Mo2(D-leu)2(L-leu)2]-Cl2(PTS)2·2H2O |
1 |
D4h |
2.114(1) |
0 |
85 |
[Mo2(O2CC5H4NH)4Cl2]Cl2·6H2O |
1 |
D4h |
2.122(1) |
0 |
77 |
Mo2(O2CNEt2)4 |
¯ |
D4h |
2.067(2) |
zero |
86 |
1 |
|||||
Mo2(glycolate)4·2H2O |
¯ |
D4h |
2.103(1) |
zero |
87 |
1 |
|||||
Mo2(O2CCPh3)4·3CH2Cl2 |
¯ |
D4h |
2.076(1) |
zero |
26 |
4 |
|||||
Mo2(R-ibp)2(S-ibp)2 |
¯ |
D4h |
2.085(2) |
zero |
88 |
1 |
|||||
ibp = ibuprofen |
|
|
|
|
|
Mo2(OSCPh)4(OPPh3)2 |
¯ |
C2h |
2.153(1) |
zero |
89 |
1 |
|||||
Mo2[O2CC6H3(OH)2]4·KCl |
2 |
D4h |
2.106(4) |
50 |
90 |
a Zero means rigorously 0; 0 means reported to be 50; 50 means not reported but apparently 50; NR means not reported and uncertain.
b The distance given in ref. 55 is in error.
There are two small groups of dimolybdenum tetracarboxylates that differ structurally from the Mo2(O2CCH3)4 model. In one group large R groups interfere with the intermolecular interactions required to form chains. This group includes Mo2(O2CCPh3)4,26 Mo2(O2CC6H4-2-Ph)4,15 and Mo2[O2C(2,4,6-PriC6H2)]4.27 The other cases where chain formation does not occur are those in which exogenous axial ligands are present, such as Mo2(O2CH)4(H2O)228 and Mo2(O2CCF3)4(py)229 and others listed in Table 4.1. In many cases, the exogenous ligands are bidentate and link the Mo2(O2CR)4 groups into infinite chains, some linear and others zigzag. In all cases, the axial Mo···L distances are very long and it must be concluded that axial bonding to the Mo2(O2CR)4 molecules is always weak. This is in contrast to the strong axial bonding to Cr2(O2CR)4 compounds.
The crystals of Mo2(OCCPh3)4·3CH2Cl226 have the Mo2(O2CCPh3)4 molecules perfectly aligned parallel to one crystallographic axis and this compound allowed the use of the polarized single-crystal visible spectrum to show definitively the location of the β Α β* absorption band.30
The minimal influence of axial coordination on the Mo–Mo bond length is best shown by comparison of the gas phase structures of Mo2(O2CCH3)431 and Mo2(O2CCF3)4,32 determined by electron diffraction, with the structures of the crystalline solids. For the acetate the distance in the isolated, gas-phase molecule, 2.079(3) Å, is c. 0.01 Å shorter than that in the crystal, 2.093(1) Å. For the trifluoroacetate, the gas and solid values are 2.105(9) Å and 2.090(4) Å. In neither case is the difference significant. The series of complexes Mo2[O2C(CH2)nCH3]4, where n = 3-9, exhibit a liquid crystalline phase between their crystalline and isotropic liquid phases.19a This liquid crystalline behavior, the first for materials incorporating M–M multiple
bonds, reflects the breakdown of intermolecular Mo···O bonding. |
|
|
|
||
For the structurally characterized Mo2(O2CR)4 compounds |
listed in |
Table |
4.1, a |
||
few |
other observations may be made. The structural |
characterization |
of the |
double |
|
salt |
{Mo2(O2CCH3)4·NaO2CCH3·HO2CCH3} shows the |
presence |
of the usual binuclear |
||

74Multiple Bonds Between Metal Atoms Chapter 4
Mo2(O2CCH3)4 unit together with pairs of hydrogen-bonded acetate/acetic acid units.33 In another structural study of Mo2(O2CCH3)4, the electron-density distribution in crystals of the acetate was determined by single-crystal X-ray diffractometry at 293K.34 The form of the defor- mation-density map was accounted for in terms of the usual representation of a quadruple Mo– Mo bond. In the case of Mo2(O2CH)4, structure determinations have been carried out on three different anhydrous forms, one orthorhombic (subsequently designated _)13,35 and two monoclinic (` and α),35 as well as on the complex Mo2(O2CH)4·KCl in which the usual Mo2(O2CH)4 molecules are linked in zig-zag chains by weak chloride bridges (Mo···Cl c. 2.86 Å) in such a way that two independent Mo2(O2CH)4 units are present.35 The Mo2(O2CH)4 molecule is also present in the ‘monohydrate’ Mo2(O2CH)4·H2O, which actually contains Mo2(O2CH)4 and Mo2(O2CH)4(H2O)2 units with Mo–Mo distances of 2.091(1) and 2.100(1) Å, respectively. In a subsequent study of the Raman and infrared spectra of Mo2(O2CH)4 and ‘Mo2(O2CH)4·H2O’, the investigators neglected to treat the hydrate as a mixture of the anhydrous and dihydrated forms.36 The pivalate complex Mo2(O2CCMe3)4 has also been structurally characterized in three different polymorphic forms.37,38
In Mo2(O2CR)4 compounds generally, the carboxyl groups are kinetically labile. Apart from occasional random observations, and the well-known fact, to be discussed in detail later, that many Mo2(O2CR)4 compounds can be prepared from the tetra-acetate by the reaction,
Mo2(O2CMe)4 + excess RCO2H Α Mo2(O2CR)4
there have been three detailed studies of the exchange process. One39 dealt with the system with Mo2(O2CH)4/Mo2(O2CCF3)4 and all four intermediates in acetone solution. At equilibrium the six species are statistically distributed, within experimental error, as shown in Fig. 4.3. It is notable that the cisoid and transoid isomers of Mo2(O2CH)2(O2CCF3)2 are present in the statistical ratio of 2:1, indicating no detectable trans influence, nor any preference for the polar cisoid isomer in the somewhat polar solvent. The rates of attainment of equilibrium at several temperatures were also measured, but not quantitatively interpreted. Equilibrium was reached in c. 30 min, beginning with a mixture of the two end members Mo2(O2CH)4 and Mo2(O2CCF3)4.
Fig. 4.3. The observed (open bars) and statistical (filled bars) percentages of the five kinds of (CF3CO2)ι (HCO2)4−ι molecules in 2:1 and 1:2 mixed solutions. The “observed” values for ι = 4 (F4) are not literally observed but calculated from the other observed values. In each case the bars for the total H2F2 are flanked by those for the cis and trans isomers.
The lability of the Mo2(O2CBut)4/Mo2(O2CCF3)4 system has also been demonstrated,40 and again the exchange processes occur rapidly (c. 1 h) at ambient temperature. In this report some suggestions were made as to the mechanism of the exchange reactions.
A kinetic study was made of the reaction between Mo2(O2CCF3)4 and NaO2CCF3 in acetonitrile.41 This reaction was monitored by19F NMR spectroscopy and, not surprisingly, the

Molybdenum Compounds 75
Cotton
data point to the existence of the adduct [Mo2(O2CCF3)4O2CCF3]− in solution. The following mechanism was proposed:41
[Mo2(O2CCF3)4O2CCF3] |
* |
|
fast |
* |
+ CF3CO2 |
|
[Mo2(O2CCF3)4O2CCF3] + CF3CO2 |
||
|
||||
* |
ks |
|
|
* |
[Mo2(O2CCF3)4O2CCF3] |
|
[Mo2(O2CCF3)3(O2CCF3)O2CCF3] |
||
|
||||
The only well-defined example of a Mo2(O2CR)4 compound with chiral carboxylates is Mo2(D(-)mandelate)4 (mandelic acid = PhCH(OH)CO2H), which was prepared by the reaction of an aqueous solution of Mo24+ (generated by admixing K4Mo2(SO4)4, Ba(CF3SO3)2 and
CF3SO3H in water) with D-mandelic acid.23 Other starting materials, specifically K4Mo2Cl8 and (NH4)5Mo2Cl9·H2O, have been used to prepare this isomer as well as the analogous derivatives with racemic and L-mandelic acid,42,43 and comparative studies have been made of the infrared42,43 and electronic absorption and CD spectra of these isomers.43,44 This work is of particular relevance to the discovery that various chiral ligands (e.g., carboxylic acids, glycols, amino alcohols, substituted thiophosphinic acids, and esters of thiophosphonic acid) coordinate to Mo2(O2CCH3)4 and that the signs of the observed CD and ORD effects of the complexes can be used to determine the absolute configurations of the ligands.45-48 While the structures of the species have not been determined, partial displacement of the acetate probably occurs in many cases.
Even when crystallographic data are not available for some molybdenum(II) carboxylates, such as the chloroacetate derivatives8 and certain insoluble bis(dicarboxylato) complexes,14 the similarity of the electronic absorption spectra and/or Raman-active ι(Mo–Mo) modes (at c. 400 cm−1) of these complexes to those of authentic quadruply-bonded complexes such as Mo2(O2CCH3)4,23,30,91,92 supports the belief that they all contain the Mo2(O2CR)4 moiety. Since a detailed consideration of the spectroscopic properties (especially electronic absorption, Raman and PES) and electronic structures of the dimolybdenum carboxylates is provided in Chapter 16, only a few of their other properties will be mentioned here. The volatility of Mo2(O2CCH3)4 and Mo2(O2CCF3)4 and the proof, via electron diffraction studies, that the dinuclear structure is retained in the vapor-phase accords with the observation that an abundant molecular ion peak [Mo2(O2CR)4]+ is seen in the mass spectra of the formate, acetate, difluoroacetate, trifluoroacetate and propionate.9,12,13,93 This property has also proved to be useful in identifying individual components in mixtures of complexes of the type Mo2(O2CC6H5)n(O2CCH2OCH3)4-n.17 The volatility of such compounds has permitted the measurement of the X-ray photoelectron spectra (XPS) and/or valence-shell photoelectron spectra (PES) of the formate, acetate, pivalate and trifluoroacetate,13,20,94,95 studies that are extremely important to an understanding of their electronic structures. The 95Mo NMR spectra of a series of Mo2(O2CR)4 compounds (R = CH3, CHCl2, CF3, Prn, Pri, Bun and But) have been recorded.96 These resonances were detected in the range 3656-4148 ppm, making them one of the most deshielded classes of molybdenum compounds so far discovered.
Two unusual Mo2(O2CR)4 species are the compounds Mo2(O2CNMe2)497 and
Mo2(O2CPBut2)4.78,79 The former complex is prepared through the insertion of CO2 into the
Mo–NMe2 bonds of triply bonded complexes of the type 1,2-Mo2(NMe2)4R2, followed by the reductive elimination of alkene and alkane:97
Mo2(NMe2)4R2 + CO2 (excess) Α Mo2(O2CNMe2)4 + alkane + alkene (R = Et, Pri, Bu)
In a related study of the reaction of CO2 with 1,2-Mo2(NMe2)4(PBut2)2 the dimolybdenum(III) complex Mo2(O2CNMe2)2(O2CPBut2)2(NMe2)2 was isolated; this converts in solution to
76Multiple Bonds Between Metal Atoms Chapter 4
the quadruply-bonded complex Mo2(O2CPBut2)4, which has been characterized by X-ray crystallography.78,79
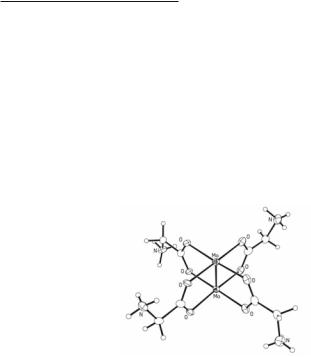
Another interesting group comprises several cationic dimolybdenum(II) species that contain amino acid ligands. The yellow glycine complex [Mo2(O2CCH2NH3)4]Cl4·nH2O was the first to be prepared80 in powder form from the reaction of glycine with K4Mo2Cl8 in hydrochloric acid. The crystalline sulfate analog [Mo2(O2CCH2NH3)4](SO4)2·4H2O is readily produced by anion exchange and a structure determination80 revealed the usual tetracarboxylato-bridged dimolybdenum unit. Because of the particular orientation of the -CH2NH3 groups, this cation is of S4 symmetry (Fig. 4.4). There are very long and weak axial interactions between each molybdenum atom and two oxygen atoms from a sulfate group.
Fig. 4.4. The core structure of the [Mo2(O2CCH2NH3)4]4+ units in [Mo2(O2CCH2NH3)4] (SO4)2υ4H2O.
Later work81 succeeded in obtaining the chloride salt in crystalline form by two methods. In the first of these, the original procedure80 was utilized but modified by using more dilute reaction solutions and employing slow mixing of an aqueous solution containing K4Mo2Cl8 and glycine with a 2 M solution of hydrochloric acid. This gave the trihydrate [Mo2(O2CCH2 NH3)4]Cl4·3H2O which had the expected structure with a Mo–Mo distance of 2.112(1) Å and very weak axial coordination (r(Mo···Cl) = 2.882(1) Å).81 An alternative and less direct method was found to give a different hydrate [Mo2(O2CCH2NH3)4]Cl4·22/3H2O.81 When Cs3Mo2Cl8H is reacted with a 1 M aqueous solution of glycine a violet species is generated which may be absorbed on a cation-exchange column.98 If the violet species is eluted with 1 M hydrochloric acid and the eluant is then stored at 0 °C under nitrogen, reduction to yellow crystalline [Mo2(O2CCH2NH3)4]Cl4·22/3H2O takes place. The structure of this hydrate has three crystallographically independent molecules, each residing on a crystallographic center of inversion (Table 4.1).
Since the initial work on these glycinate complexes, a variety of related systems have been prepared and structurally characterized. The first of these were the salts [Mo2(glygly)4]Cl4·6H2O (glygly = glycylglycine)82 and [Mo2(L-leu)4]Cl2(PTS)2·2H2O (leu = leucine; PTS = p-toluene- sulfonate),83 which were prepared by reaction of K4Mo2Cl8 with the appropriate amino acid in dilute hydrochloric acid. In both crystals, very long and weak axial Mo···Cl interactions are present. The use of this same synthetic procedure but with the racemic amino acid in place of the chiral D or L form has enabled Bino84,85 to prepare several complexes of the [Mo2(D- amino acid)2(L-amino acid)2]4+ type (amino acid = phenylalanine,84 tyrosine,84 C-phenylgly- cine,84 leucine,85 and valine85). These are listed in Table 4.1. In all instances, the four bridging amino acids ligands are coordinated to the Mo24+ unit in the cyclic order DDLL. In three of these structures, viz. [Mo2(D-leu)2(L-leu)2]Cl2(PTS)2·2H2O,85 [Mo2(D-phe)2(L-phe)2]I484 and

Molybdenum Compounds 77
Cotton
[Mo2(D-tyr)2(L-tyr)2]I4·6H2O,84 there are long weak Mo···halide axial interactions. While anhydrous complexes of stoichiometry Mo2(`-alanine)4 and Mo2(glycine)4 are said to be formed from the reactions of these acids with Mo2Cl4(PEt3)4,99 the structures of these materials have not been determined. Nicotinic acid (pyridine-3-carboxylic acid) reacts with (pyH)3Mo2Cl8H in oxygen-free hydrochloric acid to form the complex [Mo2(µ-O2CC5H4NH)4]Cl2·6H2O.77
Mo2(O2CR)4 compounds are capable of forming diadducts as first noted by Wilkinson,6 although he did not know the structures of either the adducts or their parent compounds. In general these adducts have structures of type 4.1. The ones Wilkinson made were the dipyridine adducts of the acetate and the benzoate. These two compounds readily lose the bound pyridine, and it was not until several years later that the first adduct was structurally characterized. Following the synthesis and structure determination of the trifluoroacetate Mo2(O2CCF3)4,12 its pyridine adduct was obtained upon dissolution in pyridine. The complex appears to be stable indefinitely when stored under nitrogen or argon at −20 °C. While the Mo–N distances (2.548(8) Å) are quite long, this interaction is sufficient to lead to a lengthening of the Mo–Mo bond by 0.039(6) Å relative to that in the parent Mo2(O2CCF3)4.12 In comparison to other dimolybdenum(II) complexes, the effect of axial coordination upon the metal–metal distance in Mo2(O2CCF3)4(py)2 appears to be atypically large.
|
|
|
|
R |
||||
|
|
|
|
C |
||||
|
|
O |
|
|
|
R |
||
|
|
|
OC |
|||||
|
|
|
|
O |
||||
|
|
|
|
|
|
|
O |
|
L |
Mo |
|
Mo |
|
L |
|||
|
|
|||||||
|
O |
|
O |
|
|
|
||
|
C O |
|
|
|
||||
|
|
O |
||||||
RC R
4.1
OtheradductsthathavebeenpreparedfromtheparentcarboxylatesincludeMo2(O2CCHCl2)4L2 (L = pyridine or DMSO),100 Mo2(O2CH)4L2 (L = DMSO, HCONH2, HCONMe2, HCONEt2, CH3CONMe2, CH3CONEt2, sulfolane, or tetramethylthiourea),36 Mo2(O2CCF3)4L2 (L = Me3PO or quinuclidine),101 Mo2(O2CCF3)4(AsR3)2 (R = Et or Ph),102 and the thermally unstable methanolate Mo2(O2CCF3)4(CH3OH)2.101 The trifluoroacetate Mo2(O2CCF3)4 has been reacted with the radical ligand Tempo (2,2,6,6,-tetramethylpiperindinyl-1-oxy) to form the bis(nitroxyl) radical adduct Mo2(O2CCF3)4(Tempo)2. Interestingly, magnetic susceptibility measurements show no signs of an exchange interaction down to 4.2 K.103 This is in contrast to the magnetic behavior of the dirhodium(II) Rh2(O2CCF3)4(Tempo)2, with which the molybdenum complex is isomorphous.103
Thermodynamic data have been obtained from calorimetric measurements on toluene solutions of several 1:1 and 2:1 adducts of Mo2(O2CC3F7)4 with CH2CN, py, DMSO, DMA, etc.104 A comparison of these data with those for the analogous dirhodium(II) complexes indicates that the Mo2(O2CCF3)4 is the weaker Lewis acid.
Of the neutral 1:2 adducts that are known, those involving phosphine ligands are the most interesting. Although the adduct Mo2(O2CCF3)4(PPh3)2 has been known since the early 1970s,22,101 it was not until 1980 that the first detailed study of the reactions of a dimolybdenum(II) carboxylate with a wide range of phosphine ligands was reported.105 Based upon a combined 1H, 19F and 31P NMR and infrared spectral study on the adducts Mo2(O2CCF3)4(PR3)2, it was concluded that they fall into two structural classes.105 Some possess a structure of the type shown in 4.1 (L = PPh3, P(C6H11)3, PBut3 and P(SiMe3)3). 31P{1H} NMR spectroscopy showed that these adducts are extensively dissociated in CDCl3 solution.105 Other complexes, which are

78Multiple Bonds Between Metal Atoms Chapter 4
formed with PMe3, PMe2Ph, PEt3, PEt2Ph and PBun3,22,56,105 have structures in which there are both bidentate bridging and monodentate trifluoroacetate groups as represented in 4.2. The complex Mo2(O2CCF3)4(PMePh2)2 is unusual in existing in both structural forms; this has been confirmed by crystal structure determinations on the orange-yellow (4.1)57 and red-orange (4.2)56 isomeric forms (Table 4.1). X-ray crystal structures have also been reported for the 4.2 type complexes Mo2(O2CCF3)4(PEt2Ph)256 and Mo2(O2CCF3)4(PBun3)2.22 The structure of the type 4.1 complex Mo2(O2CCF3)4(PPh3)2 has also been determined,56 but not fully refined. The spectroscopic properties of the arsine complexes Mo2(O2CCF3)4(AsR3)2 (R = Et or Ph) are consistent with structure type 4.1.102
R
C
OO
|
|
|
PR3 |
O2CR |
|
|
|
|
|
||
RCO2 |
Mo |
|
Mo |
||
|
|||||
|
|
R3P |
|
||
|
O |
|
O |
||
|
|
|
C |
||
|
|
|
R |
||
4.2
A criterion for predicting which phosphine adduct will have which structure has been developed. This is based on a cone angle versus basicity relationship: type 4.2 complexes are formed only by the smallest and most basic phosphines.105 In a related context, it has been reported106 that the predominant species in pyridine-containing solutions of Mo2(O2CCF3)4 is the 1:4 adduct Mo2(O2CCF3)4(py)4 and not Mo2(O2CCF3)4(py)2, the latter being isolated upon crystallization. The former complex is believed to have a structure in which two of the trifluoroacetate groups are monodentate. This result indicates that the type 4.1/4.2 structural behavior may not be restricted to phosphine ligands.
Although the aforementioned 1:2 adducts are most easily prepared by the direct reaction of the phosphine with the dimolybdenum(II) carboxylate, other procedures are possible. Thus, the benzoate complex Mo2(O2CC6H5)4(PBun3)2 has been obtained by the treatment of Mo2Br4(PBun3)4 with benzoic acid (1:4 mole proportions) in refluxing benzene.107 Also, mixed phosphine complexes can be formed, as in the case of Mo2(O2CCF3)4(PEt3)( PBun3) which is produced when equimolar quantities of Mo2(O2CCF3)4(PEt3)2 and Mo2(O2CCF3)4(PBun3)2 are mixed in toluene at −80 °C.22
While some bidentate phosphines (Me2PCH2PMe2, Ph2PCH2PPh2 and Me2PCH2CH2PMe2) react with Mo2(O2CCF3)4, the products remain less well characterized than adducts with monodentate phosphines, although in some of them monodentate trifluoroacetate groups and chelating phosphine ligands may be present.105 In contrast, the polymeric acetate complex [Mo2(O2CCH3)4(µ-dmpe)] and its amine analog [Mo2(O2CCH3)4(µ-tmed)] are well characterized.49 In each, there are infinite zig-zag chains of Mo2(O2CCH3)4 linked by the bridging Me2ACH2CH2AMe2 ligands (A = N or P). Both have structures of type 4.1. With the complex [Mo2(O2CCH3)4(µ-dmed)] (dmed = MeNHCH2CH2NHMe, the chain structure is kinked due to hydrogen bonding effects.108 Polymeric chain compounds [Mo2(O2CCH3)4(µ-L)] have also been prepared and characterized in the case of L being pyrazine, 4,4'-bipyridine, and 1,4- diazabicyclo[2.2.2]octane.51
The use of the phosphine adducts Mo2(O2CR)4(PR3)2 as precursors to other dimolybdenum compounds has scarcely been examined. The one exception is a report that Mo2(O2CCF3)4(PR3)2 (R = Et or Ph) act as templates for the self condensation of 2-aminobenzaldehyde to give dimolybdenum species that contain a tetradentate macrocyclic ligand.109 The structures of these complexes remain to be definitively established.
