
Metal-Catalysed Reactions of Hydrocarbons / 03-Chemisorption and Reactions of Hydrogen
.pdf
3
CHEMISORPTION AND REACTIONS OF HYDROGEN
PREFACE
The hydrogen molecule is dissociatively chemisorbed with great facility by a number of metals within the Transition Series: elsewhere the process is more highly activated, but chemisorbed atoms once formed are stable. The contrast between hydrogen and the alkanes is very striking: the σ H H bond can sometimes be broken at temperatures close to 20 K, the activation energy being minimal, whereas with methane each σ C H bond is effectively shielded by the other hydrogen atoms, so that much higher temperatures are needed for its chemisorption by dissociation.
The strengths of hydrogen-metal bonds at the surfaces of metals of Groups 8 to 10 are of similar order, but they tend to increase on moving towards the centre of each Transition Series. These trends are shown more distinctly by other molecules such as oxygen and nitrogen, where a clear parallelism with analogous bulk compounds is shown: but within the Transition Series stoichiometric hydrides are formed only by metals of Groups 3, 4 and 5. Because of commercial interest and experimental convenience, certain metals have commanded disproportionate attention: tungsten, platinum and nickel in the massive form and platinum also in the microscopic form. For these same reasons, other metals have escaped attention almost entirely (e.g. manganese and osmium).
All the catalysed reactions to be considered in the following chapters involve hydrogen (or deuterium) as reactant or product, and a knowledge of its chemisorption, and of reaction between its isotopic and spin variants, will prove helpful in understanding its reaction with hydrocarbons. For this reason most emphasis will be placed on the interaction of hydrogen with metals of Groups 8 to 10.
While the utility of hydrogen in catalysed reactions is due in part to its small size and easy migration, these features add greatly to the difficulty of describing
93

94 |
CHAPTER 3 |
the process of adsorption and desorption and the structure of the adsorbed layer under any specified conditions, especially when the adsorbent is a supported metal.
3.1. THE INTERACTION OF HYDROGEN WITH METALS
As almost all the catalysed reactions to be considered in the following chapters involve hydrogen (or deuterium) as reactant or product, it is first necessary to explore how this molecule interacts with metals in general, and those metals of most interest to catalysis in particular.1−17 The hydrogen-metal system has been extremely thoroughly studied, and a whole book has been devoted to the relevant surface chemistry and catalysis. The interactions that occur are also of great importance in metallurgical processing, but that is beyond the scope of this work.
Hydrogen interacts with metals in three principal ways: (i) by dissociative chemisorption at the surface; (ii) by physical adsorption as molecules at very low temperatures; and (iii) by dissolution18 or occlusion.19,20 As we shall see, to these three extreme forms have been added numerous intermediate states of various lifetimes and stabilities, some of which may have importance in catalysis. There is for example clear evidence for a molecular state formed at about 100 K on stepped surfaces saturated with atomic hydrogen (on Ni(510),21,22 Pd(510)21 and (210)23): this is distinct from a molecular precursor state such as that seen with deuterium on Ni(111) at 100 K. The role of such states will be discussed further below (Sections 3.2.2 and 3.3.3). The small size and electronic simplicity of the hydrogen atom formed by dissociation enable it to bond to metal surfaces in different ways, and simple-minded notions about its forming only a single covalent bond to another atom have to be abandoned.
The earliest studies of the chemisorption of hydrogen by metals were made using powders, foils and wires, which were polycrystalline, exposing various crystal planes, and by today’s standards far from clean.24 Nevertheless this early work started to delineate some of the main features of the interactions. During the late 1940s, Otto Beeck and his associates at the Shell Development Company developed the use of metal films25 (Section 1.2.1), and these were extensively used in the following decades for studies of chemisorption and catalysis.26–28 Although still polycrystalline, it was possible to persuade them to expose a specific crystal plane preferentially,29 and bimetallic (alloy) films were also made. Such films had quite clean surfaces and yielded results that have been useful for correlating strength of adsorption with catalytic activity.30 The next important development was field-emission microscopy (FEM),31 followed by field-ion microscopy (FIM)26,32–34 (Section 1.2.1), techniques which revealed the preference of adsorbates for different crystal planes at the tip of very fine needles, albeit under stressful conditions.31,35 The most recent advance has been the use of single crystal metal

CHEMISORPTION AND REACTIONS OF HYDROGEN |
95 |
surfaces,36–38 exposing chiefly a single crystal plane or a predetermined variety of configuration on stepped or kinked surfaces37,39,40 (Section 1.21). Their surfaces may be thoroughly cleaned by ion bombardment and identified by LEED, although initial cleanliness is not always maintained (e.g. dissolved impurities such as sulfur may emerge slowly) and STM reveals defects and imperfections that LEED misses. Nevertheless their employment has caused a revolution in our understanding of the dynamics of formation and structure of chemisorbed layers. This information, some aspects of which will be summarised in the next section, is of limited relevance to the behaviour of small metal particles, although pertinent to those catalysed reactions that can be observed on single crystals: the degree of relevance is increased by using stepped and kinked surfaces.
The way in which hydrogen interacts with supported metal catalysts is necessarily more complex than is the case with single crystals. Small particles expose different crystal planes and many edge and corner atoms, and are probably more defective than massive forms (Section 2.5.2). Moreover, although hydrogen only rarely interacts directly with the materials commonly used as supports, it can nevertheless migrate with surprising ease from metal to support, producing a number of interesting and occasionally useful consequences (Section 3.34). Reaction at the metal-support interface has also been detected. The frequent use of hydrogen chemisorption and of hydrogen-oxygen titration to estimate metal area and particle size41 is justified by the low cost and simple operation of these techniques, but great experimental and theoretical care is needed before all ambiguities and uncertainties are removed, and complete confidence in the answer is obtained.
The physical adsorption of molecules at surfaces is due to dispersion or Van der Waals forces of the type that hold them together in the liquid state, so that this form is not in general stable much above the boiling point of the liquid. In the case of hydrogen, its physical adsorption at ambient temperature and above can safely be ignored, although its existence in principle provides a means of bringing the molecule close to the surface without dissociation, thus easing the breaking of the bond (Section 3.2.1).
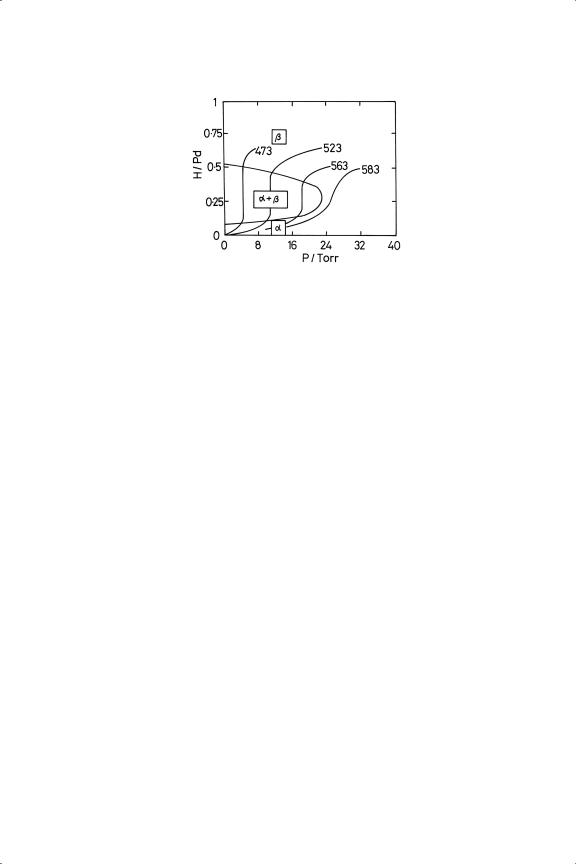
Hydrogen dissolves into a number of the Transition Series metals with the formation of metallic hydrides;6,11,14,42–44 with platinum however, the process is endothermic and occurs only to a small extent and is therefore unimportant. Apart from palladium, only in the case of iridium has its retention or occlusion within the grain structure been implicated in catalytic behaviour, being thought responsible for the low selectivity which this metal usually gives in the hydrogenation of alkynes and alkadienes19,20 (Chapters 8 and 9). Palladium is however unique in its capacity to absorb hydrogen;6,14,43,45–47 on raising the pressure at room temperature, the α-phase is first formed, and it co-exists with the β-phase in the H/Pd range 0.05 to 0.58, after which only the β-phase exists48 (Figure 3.1). The range of
96 |
CHAPTER 3 |
Figure 3.1. Phase diagram for the palladium-hydrogen system (note the resemblance to the CO2 phase diagram).
co-existence of the two phases decreases with increasing temperature. The β-phase is a semiconductor, but mechanical strength is maintained to quite high H/Pd. A major application of this property is to produce hydrogen of high purity,49 by diffusion through a palladium sheet or membrane: it is preferable to use an alloy with silver (Pd77Ag23) to avoid mechanical failure through embrittlement due to passage in and out of the β-phase, the formation of which causes a volume expansion of 10%. Much significant catalytic work has been done with palladium alloys of the Group 11 metals,49,50 and the solubility of hydrogen and deuterium in them has been investigated.47,51–53 The concentration of hydrogen atoms in palladium at saturation corresponds to about Pd4H3 and approaches that in liquid hydrogen. It now appears however that such a concentration is insufficient to sustain nuclear fusion at ordinary temperatures when hydrogen is replaced by deuterium. Solubility decreases as particle size becomes smaller,15,44,54,55 and reduction of small palladium oxide particles by hydrogen gives metallic palladium, while larger particles lead to the hydride phase. There is circumstantial evidence to show that hydrogen atoms can dissolve into and diffuse quite easily through gold;56−58they also dissolve into nickel59 and nickel-copper alloys60 under electrochemical persuasion. The extraordinary properties of the palladiumhydrogen system have been thoroughly studied, and are described in an extensive literature.15,46,48
Earlier elements in the Transition Series (Groups 1 to 5) and intermetallic compounds containing a Rare Earth element (e.g. LaNi5) form stoichiometric hydride phases which have possible application in hydrogen storage, but they are loath to release their hydrogen to acceptor molecules and even in the presence of hydrogen gas are undistinguished as catalysts.15 Many of the preand postTransition Series elements form molecular hydrides,43 some of which (notably elements in Groups 3, 4 and 14) are polymeric.

CHEMISORPTION AND REACTIONS OF HYDROGEN |
97 |
3.2.CHEMISORPTION OF HYDROGEN ON UNSUPPORTED METALS AND ALLOYS61,62
3.2.1. Introduction
The nucleus of the hydrogen atom (1H) is a single proton; addition of one neutron gives a deuteron (D or 2H, natural abundance 0.0156%), and of two neutrons gives a tritium atom (T or 3H): the D nucleus is stable, but the T nucleus is a weak β-emitter (half-life 12.35 years). The diatomic molecules HD, H2 and D2 differ significantly in their mass and other physical characteristics, making analysis of their mixtures quite straightforward, and the mass distinction in particular has allowed deuterium used as a stable isotopic tracer for more than half a century; tritium atoms are easily located by standard radiochemical methods. Isotopically pure samples are hard to obtain, and in admixture species such as hydrogen deuteride (HD) or hydrogen tritide (HT) will prevail. The equilibrium constant for the system
|
2HD |
(3.A) |
H2 + D2 |
is about 3.2 at ambient temperature, due to the zero-point energy effect. Nuclear spin isomers also exist, in which the spins are either parallel or anti-parallel: parallel spins give the ortho-forms, which at equilibrium constitute 75% of hydrogen and tritium, and 66.7% of deuterium, while the anti-parallel spins give the para-forms. The stability of the hydrogen molecule (dissociation energy 436 kJ mol−1) is such that atoms only exist at equilibrium with it to very small extents (0.08% at 2000 K). Dissociation is achieved more easily by mercury-sensitised photolysis and especially by metallic and other catalysts. Interconversion of spin isomers is not diagnostic for dissociation and recombination because this can be induced by a strong magnetic field; equilibration of molecular hydrogen and deuterium, forming hydrogen deuteride of course demands bond breaking and re-formation. Basic studies of chemisorption are usually made with hydrogen, although subtle but significant differences are sometimes seen when isomers are compared.63,64
Most of the results to be reviewed in this section will have been obtained on single crystals cut to exposed a defined place which may be flat, stepped or kinked and on surface alloys: where necessary (because of the lack of information on the relevant single crystal), or helpful for purposes of comparison, we shall refer to results obtained on polycrystalline forms (films, powders etc). This attention to single crystals is justified by the reliability and fundamental nature of the measurements, which involve a large number of sophisticated surface-sensitive techniques or procedures for monitoring the solid state as it responds to chemisorption at its surface. Many of the methods referred to in the preceding Chapter for characterising clean surfaces also provide information on hydrogen chemisorption, some of the more important including LEED and related methods, 1H and 129Xe

98 |
CHAPTER 3 |
NMR, EXAFS/XANES,65,66 and STM67 (see Section 2.4.2). Procedures specific to chemisorbed states3,17,29,30,68–70 include measurement of changes in electrical or magnetic character, vibrational spectroscopies, (HREELS, DRIFTS/RAIRS etc), calorimetry71 and thermal desorption.72,73 This short list is far from being comprehensive, and the reader is reminded that the purpose of this work is not to instruct in the use of these techniques, but rather to present and evaluate the results they generate. The principles concerned are only mentioned where understanding of the results necessitates it. This is somewhat in the spirit of Jerome K. Jerome, who in his Preface to ‘Three Men in a Boat (to say nothing of the dog)’ advised his readers not to use it as a manual for a River Thames holiday; they would, he said, be wiser to stay ay home.
It is not easy to decide how to order a summary of the vast amount of information available. The theme which we seek to illustrate is how the processes of chemisorption and desorption and the properties of the adsorbed state depend upon the chemical composition of the surface and its atomic structure. The rest of this section (3.2) is sub-divided into the chemisorption process (3.2.2), structural aspects of the chemisorbed state (3.2.3) and energetic aspects including desorption (3.2.4). Such subdivision is highly artificial as there are close interrelations between all subsets; however, each technique is chiefly directed towards obtaining information of a specific type, and integration of the various results is largely an intellectual or modelling exercise.
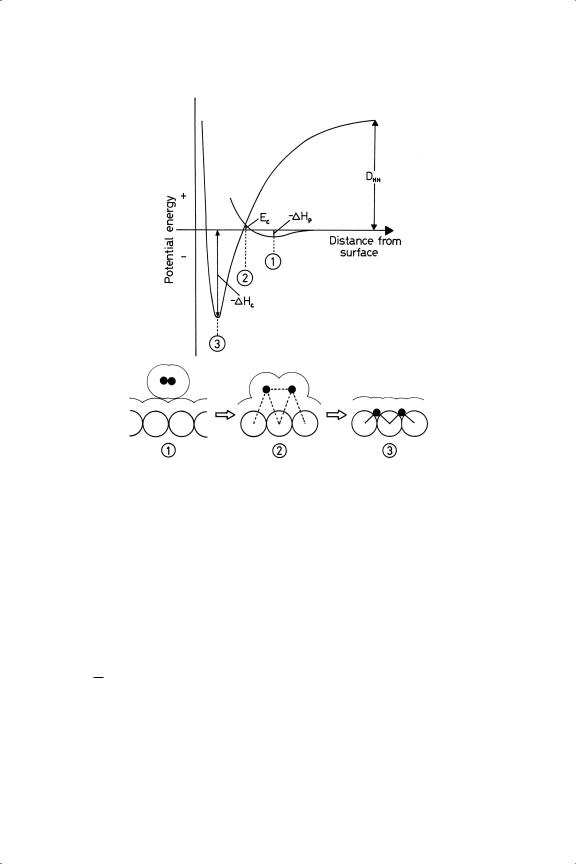
A convenient way of entering the detail of the subject is through the one dimensional potential energy diagram due originally to Sir John Lennard-Jones:74 it is one dimensional in the sense that the H M distance is the only variable considered, whereas the H H distance will also increase as dissociation occurs and a fuller representation of the process is possible by two-dimension diagrams containing both distances as variables.3,75 The basic one-dimensional picture3,6 (Figure 3.2) depicts a molecule approaching the surface and falling into a shallow energy trough, the depth of which gives the heat of physical adsorption (typically 10–20 kJ mol−1); the minimum is quite far from the surface, at a distance equal to the sums of the covalent and Van der Waals radii of the metal and hydrogen atoms. This process is not activated and this state would not be significantly occupied at ordinary temperatures.
The second curve is for two separate atoms coming towards the surface: it therefore starts at the right well above the energy zero, as 436 kJ mol−1 have to be found to atomise the molecule in free space. The depth of the trough gives the heat of chemisorption and the minimum is quite close to the surface, the distance being roughly the sum of the covalent radii. In Figure 3.2 the curves are drawn so that the transition state occurs very close to this potential energy zero, which means that the process of chemisorption shown here has only a very small activation energy. It does however have a finite value, because the chemisorption of deuterium is slower than that of hydrogen at 87 K, the rate being sensitive to the zero-point energy
CHEMISORPTION AND REACTIONS OF HYDROGEN |
99 |
Figure 3.2. Lennard-Jones potential energy diagram for the interaction of hydrogen with the surface of a metal of Groups 8–10 (see text for description). The lower part of the diagram shows possible configuration at three points in the chemisorption process.
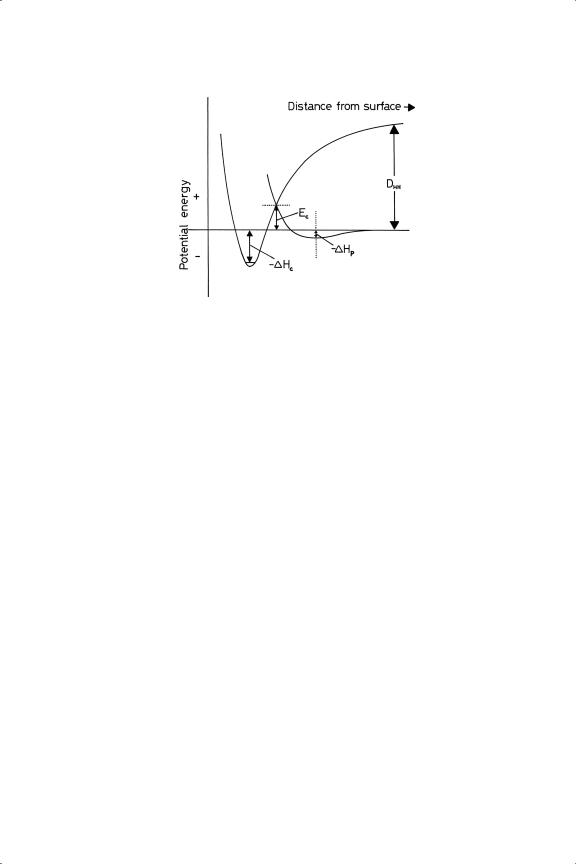
difference. Small activation energies are the general rule for metals of Groups 8 to 10 (Ec < 10 kJ mol−1), but for the coinage metals in Group 11 they are large and quite easily measurable76 (Ec 20–60 kJ mol−1). We can see that the state of physical adsorption provides a pathway for a molecule to approach the surface and to attain a position where interaction of its orbitals with those of the metal can proceed and lead to dissociation, without a disconcertingly large input of energy; therein lies much of the secret of catalysis. Some other forms of energy (thermal, radiative etc) can also induce dissociation, but it is the great strength of the H H bond that determines that in the absence of a catalyst it can be broken only at a very high temperature. The presence of a catalyst is therefore essential for reactions involving hydrogen to proceed under mild conditions. Figure 3.3 shows the intervention of a significant activation energy in the case of Group 11 metals.
The potential energy diagram can be extended to show features of the process of dissolution of hydrogen atoms into the bulk.3 There is evidence in some systems
100 |
CHAPTER 3 |
Figure 3.3. Potential energy diagram for the interaction of hydrogen with the surface of a metal of Group 11.
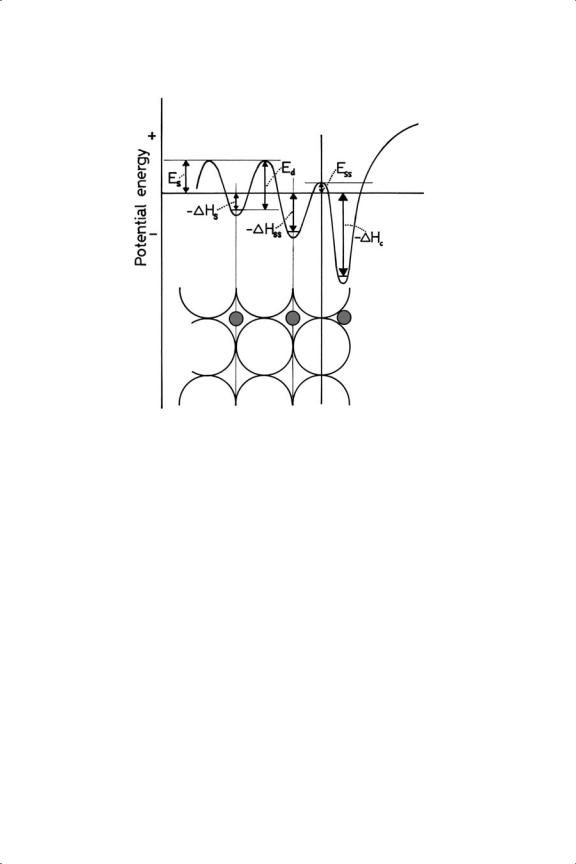
that sites immediately below the surface atoms can accommodate them in a stable way,77,78 whence (especially with palladium and its alloys) they migrate into the interior. Figure 3.4 shows the heat content changes and activation energies involved.
These diagrams are helpful in understanding how chemisorption of hydrogen occurs, but they have their limitations: they imply for example that all hydrogen atoms are chemisorbed in the same way and with the same strength, but this as we shall see is rarely true. The initial collision may, for example, give atoms at places where they are ‘uncomfortable’ and from which they may diffuse to more energetically favourable positions. This initial condition constitutes an atomic precursor state.4
3.2.2. The Process of Chemisorption26,32,33,68,75,78
The fundamental measure of the reactivity of a metal surface towards hydrogen is the initial sticking probability So defined simply as the fraction of molecules colliding with the surface that remain chemisorbed, extrapolated to zero coverage. As the exposure (defined as pressure × time) increases, so does the coverage θ , and the sticking probability S must fall; if a molecular beam gas source is used, the increase of the background pressure (measured by an ion gauge or a mass spectrometer) after a particular exposure, ratioed to the pressure rise when no further adsorption occurs, gives the value of S at that point. The commonly used unit of exposure is the Langmuir, which is 10−6 Torr s; one Langmuir (1 L) will give a full coverage of a typical metal surface if S is unity. Coverage is calculated from the number of molecules that have chemisorbed and the estimated number of surface atoms.
CHEMISORPTION AND REACTIONS OF HYDROGEN |
101 |
Figure 3.4. Potential diagram showing the process of dissolution of hydrogen atoms into a metal: ss = subsurface site; s = dissolution; d = diffusion.
It is worth recalling from Section 1.2.2 that a surface is defined by the crystallographic plane in which the outermost atoms reside, although with certain atomically rough or ‘open’ surfaces, such as the fcc(110) or the bcc(111), atoms from a second or even a third underlying layer are also substantially exposed. It is usual to express coverage in terms only of the atoms in the strict surface plane and the result is that with such surfaces, values of the coverage θ may rise well above unity since the partly exposed atoms also contribute to the bonding capability of the surface. Absolute coverages of deuterium on Pt(111) have been measured79 by nuclear microanalysis using the reaction D(3He,p)4He.
The sticking probability S of the hydrogen molecule being the simplest measure of metal surface reactivity, its dependence upon crystal face and coverage may provide ideas for understanding other reactions. In the absence of a precursor state and on an energetically homogeneous surface S will depend upon θ as
S = S0(1 − θ )n |
(3.1) |
where n is the number of atoms making up the site, but where a precursor state exists S will initially fall less quickly, but more rapidly as the coverage approaches completion. The initial value S0 is determined by the temperature of the surface,

102 |
CHAPTER 3 |
by the translational and internal energies of the molecule as it hits it, and by its chemical nature and structure. Much information has been obtained on the effect of these variables in the case of the Group 11 metals64,80 and their alloys,81 where the existence of a significant activation energy Eads (see Figure 3.3) and a low value of S0 makes experimentation more straightforward than with metals of Groups 8 to 10. Surface temperature matters when some intermediate adsorbed state exists for sufficiently long to attain thermal equilibrium, but not if dissociation is a direct, activated process. Excitation of both translational and vibrational energies of the colliding molecule assists dissociation, but rotational energy seems to have little effect. The different behaviours shown by hydrogen and deuterium help to resolve details of the process of dissociation.63,64
In the case of the Groups 8 to 10 metals, a major factor is the surface structure.62 With the atomically compact surfaces such as the fcc(100) and (111) values of S0 are usually small (<0.1), but with the more open fcc(110) and cph(1010) the incoming molecule experiences a softer and deeper potential well (and a greater variety of emergent orbitals) and So values are close to unity.1,17,75 Their roughness may also help to dispose of the heat of adsorption, which is probably used to excite lattice phonons (i.e., vibrational modes of the solid).1,75 The literature states that defects, and intentional irregularities such as steps and kinks, encourage sticking,82 but in the case of platinum this has been disputed;83 preadsorbed oxygen on Pt(110) lowers the sticking coefficient of hydrogen,84 and the effect of potassium on Ni(111)85and on Pt(111) is similar.86 There is clearly much yet to be learned about this seemingly simple process.75
There is a useful application of the Principle of Microscopic Reversibility (or Detailed Balancing) in the study of surface processes. This is a principle that requires that, when carried out under identical conditions, the reverse of any process should proceed by exactly the same route as the forward process: thus whatever energy input is needed for the chemisorption of a hydrogen molecule will be recovered and released when the two atoms recombine and desorb. Measurement of the relaxation of the vibrational and translational energy of the desorbing molecule therefore provides information on the needs in dissociation , and values of S can also be derived.80
As we shall see in what follows, in very many cases there are distinct ‘phases’ formed as the coverage by hydrogen atoms increases; these show characteristic values of heat of adsorption, sticking probability S and other physical properties. The fine structures of S versus θ plots that are observed when the process of chemisorption is not energetically uniform will be considered in the following section.
3.2.3. The Chemisorbed State: Geometric Aspects1,3,17,75
We have seen that the kind of metal used and the atomic structure of its surface both affect the ease with which the hydrogen molecule becomes dissociatively
