
Metal-Catalysed Reactions of Hydrocarbons / 01-Metals and Alloys
.pdf
1
METALS AND ALLOYS
PREFACE
This book in some ways resembles a detective story, but the criminal that we seek is the answer to the question: which solid-state properties of metals determine their behaviour as catalysts for the reactions of hydrocarbons? The search will lead us from the bulk metallic state through the small supported metal particles whose greater area makes them more fit to catalyse in a useful way; and from the reactions of hydrogen and hydrocarbon molecules with both sorts of metal to their catalytic interactions. The chain of cause and effect may not be straightforward.
In the metals of the Transition Series, where our attention will be focused, the strength of interatomic bonding and all the parameters which reflect it vary greatly: only six nuclear charges and their compensating electrons separate tungsten from mercury. The clear physicochemical differences that separate the metals of the first Transition Series from those in the second and third Series will be reflected in their chemisorptive and catalytic properties, as will the subtler differences between the second and third Series, for the understanding of which we are indebted to Albert Einstein and Paul Dirac. Gold has always been seen as the ultimate in nobility and iron as most liable to corrode; indeed this contrast was invoked by Geoffrey Chaucer’s village priest, who in describing the high qualities needed in one of his calling, asked rhetorically If gold rust, what shall iron do?
Metal surfaces are the place where chemical changes start and even on large pieces of many metals the surface atoms are not quiescent but in a state of permanent agitation; this has quite a lot to do with their reactivity. They are, as Flann O’Brien remarked, livelier than twenty leprechauns dancing a jig on a tombstone.
While there are only some seventy-five metals, there are an infinite number of binary alloys, and it is little wonder that some are better catalysts than the pure metals that comprise them. In telling the story of catalysis by alloys we shall see how suspects were wrongly identified, and how the real truth was discovered: but
1
2 |
CHAPTER 1 |
first we have to know something about the structure of metals and the metallic surface.
1.1. THE METALLIC STATE
1.1.1. Characteristic Properties
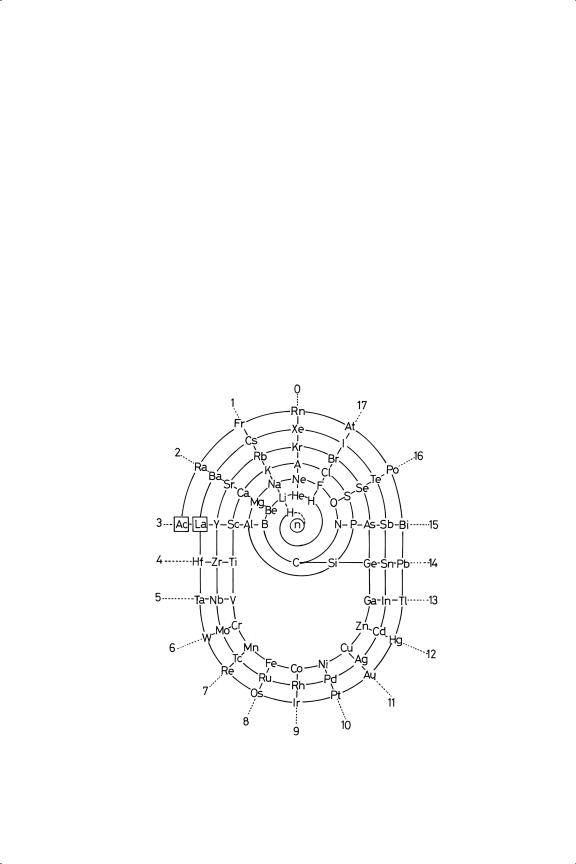
Of the first one hundred elements in the Periodic Table (Figure 1.1), about seventy-five are metals: in bulk form, most of them exhibit the characteristic physical properties of the metallic state, namely, strength, hardness, ductility, malleability and lustre, as well as high electrical and thermal conductivity. They owe their chemical and physical properties to their having one or more easily removed valence electrons: they are therefore electropositive, and most of their inorganic chemistry is associated with their simple or complex cations.1,2 Metallic character in certain Groups of the Periodic Table increases visibly with increasing atomic number: while all the d-block elements in Groups 3 to 13 are obviously metals,
Figure 1.1. Periodic Classification of the elements.

METALS AND ALLOYS |
3 |
of the Groups containing elements of the short Series, i.e. the sp-elements, this is only true of Group 1 (Figure 1.1). In Groups 2, 3, 14 and 15, the early elements are either clearly non-metallic or are semi-metals (e.g. beryllium, boron). The transition from non-metallic to semi-metallic to wholly metallic behaviour is most evident in Group 14, in which silicon and germanium are semi-metals, tin is ambivalent (the grey allotrope, α-Sn, is a semi-metal, while the much denser white form (β-Sn) is metallic), and lead is of course a metal. In Group 15, arsenic and antimony are semi-metals, but bismuth is a metal; in Group 16, tellurium and polonium are semi-metals.3
It is however not always easy to decide what substances show metallic behaviour.4−6 One criterion for distinguishing semi-metals from true metals under normal conditions is that the co-ordination number of the former is never greater than eight, while for metals it is usually twelve (or more, if for the body-centred cubic structure one counts next-nearest neighbours as well). Other criteria have been proposed. Which category an element falls into also depends upon the conditions employed; thus for example some metals lose their metallic character above their critical temperature (e.g. mercury) or when in solution (e.g. sodium in liquid ammonia). Interatomic separation is then large and valence orbitals cannot overlap, so electrical conduction is impossible. On the other hand, the application of pressure causes some substances that are normally insulators or semiconductors to behave like metals; thus for example α-Sn changes into β-Sn, in accordance with Le Chatelier’s Principle.2 Similar changes also occur with other semi-metals (e.g. silicon and germanium), and even hydrogen under extreme pressure shows metallic character. Electrical conduction takes place when metal atoms are close enough together for extensive overlap of valence orbitals to occur. All metals when sufficiently subdivided fail to show the typical characteristics of the bulk state; the question of the minimum number of atoms in a particle for metallic character to be shown will be considered in Chapter 2.
The physical and structural attributes of the metals vary very widely: tungsten for example melts only at about 3680 K, while mercury is a liquid at room temperature (m.p. 234 K), this change being produced by increasing the nuclear charge only by six. The way in which the outermost electrons are employed in bonding ultimately determines all aspects of the metallic state. This is a question which is poorly treated if at all in text books of inorganic chemistry,1,2,7 so some further description of the relevant facts and theories will be necessary. This information bears closely on the chemisorptive and catalytic properties of metal surfaces, which are our principal concern. When a metal surface is created by splitting a crystal, bonds linking atoms are broken, and in the first instant the dangling bonds or free valencies thus formed have some of the character of the unbroken bonds. We may therefore expect to see some parallelism between the behaviour of metals as shown by the chemical properties of their surfaces and the manner in which their valence electrons are used in bonding.

4 |
CHAPTER 1 |
The metals of interest and use in catalysis are confined to a very small area of the Periodic Table, so that most of our attention will be given to the nine metals in Groups 8 to 10,8 with only occasional mention of neighbouring elements in Groups 7 and 11, and of the earlier metals of the Transition Series (Figure 1.1). A principal object of our enquiry will be to understand why catalysis is thus restricted and our gaze will therefore be limited largely to the trends in metallic properties that occur in and immediately after the three Transition Series.
The properties of metals that disclose how their valence electrons are used may be put into four general classes: (i) mechanical, (ii) geometric, (iii) energetic, and (iv) electronic. The mechanical class (hardness, strength, ductility and malleability) may be quickly dismissed, because in polycrystalline materials these are mainly controlled by interactions at grain boundaries, and are influenced both by adventitious impurities that lodge there, and by deliberate additions that result in grain stabilisation, with consequent improvement in strength and hardness. With single crystals, they are described by the plastic and elastic moduli, which in turn are governed by the ease of formation and mobility of defects within the bulk under conditions of stress. They bear some relation to the strength of metallic bonding, but are of lesser interest than other properties. Metals having the body-centred cubic structure are less ductile than those that have close-packed structures (see below), because they lack the planes of hexagonal symmetry that slide easily past each other.
Bulk geometric parameters are those that describe the arrangement of the positive nuclei in space, and the distances separating them: the former is conveyed by the crystal structure and co-ordination number, and the latter by the metallic radius. Most metals crystallise in either the face-centred cubic (fcc) or the close-packed hexagonal (cph) or the body-centred cubic (bcc) structure; the first two are alternative forms of closest packing (Figure 1.1). Four other structures are known: rhombohedral (distorted fcc: mercury, bismuth), body-centred tetragonal (A4, e.g. grey tin9), face-centred tetragonal (indium, manganese), and orthorhombic (distorted cph: gallium, uranium). Many metals exhibit allotropy, i.e. they exhibit different structures in different regimes of temperature but in catalysis our only concern is with the form stable below about 770 K; of the metals of catalytic interest, only cobalt suffers a phase transition below this temperature (from cph to fcc at 690 K). Within the Transition Series there is a strikingly regular periodic variation in crystal structure; most metals in Groups 3 and 4 are cph (aluminium is fcc), those in Groups 5 and 6 are bcc, those in Groups 7 and 8 are again mainly cph (excepting iron, which is bcc, and manganese), while those in Groups 9 to 11 (except cobalt) are all fcc at ordinary temperatures.3,10 Explanation of this regularity will be a prime requirement for theories of the metallic state (Section 1.12).
As the nuclear charge increases on moving across each Transition Series, the number of valence electrons forming covalent bonds at first rises rapidly, then
METALS AND ALLOYS |
5 |
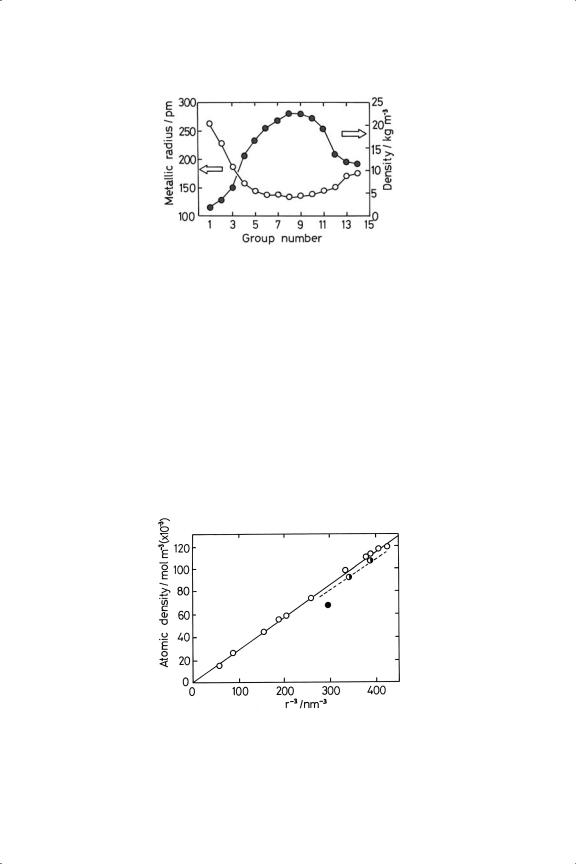
Figure 1.2. Periodic variation of metallic radius and density in the Third Transition Series.
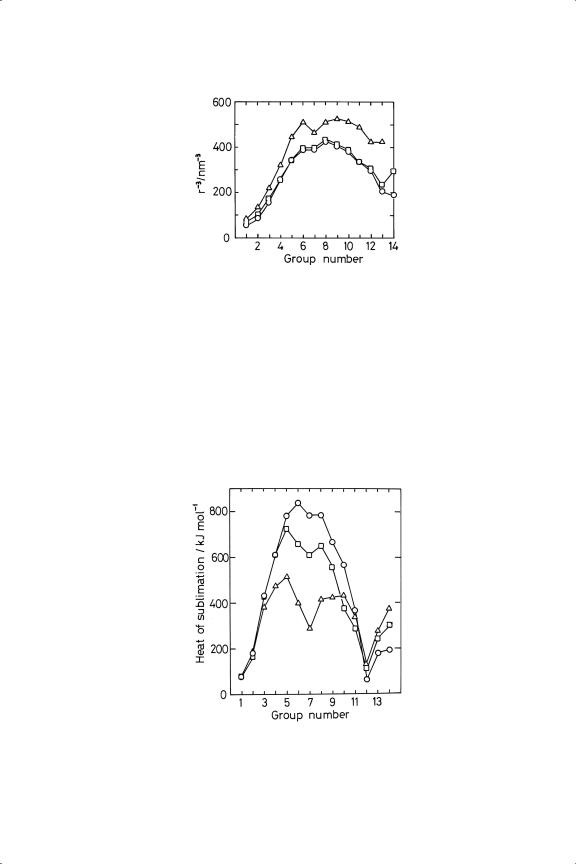
remains almost constant in Groups 5 to 10, and afterwards starts to fall. This effect is clearly shown in metallic radius and density, values for which for the third Transition Series are shown in Figure 1.2. To relate these two parameters precisely, it is necessary to correct for changes in atomic mass. The plot of atomic density (i.e. density/atomic mass) versus the reciprocal of the cube of the radius (Figure 1.3) shows two good straight lines, one for the close-packed metals and another of slightly lower slope for the more open bcc metals. Figure 1.4 shows the periodic variation of the reciprocal cube of the radius for all three Transition Series: in the First Series iron, cobalt and nickel have almost the same bond lengths, while in the later Series the minimum bond length is shown at ruthenium and osmium. The similarity between the bond lengths in the second and third Series is only partly a consequence of the Lanthanide Contraction (see below).
Figure 1.3. Dependence of atomic density on the reciprocal of the cube of the radius for metals of the Third Transition Series; open points, close-packed structures; half-filled points, bcc structure; filled point, Hg.
6 |
CHAPTER 1 |
Figure 1.4. Periodic variation of the reciprocal of the cube of the radius for metals of Groups 1 to 14; triangles, First Series; squares, Second Series; circles, Third Series.
While geometric parameters reflect indirectly the strength of bonds between atoms, a more direct approach is provided by energetic parameters relating to phase change, i.e. melting and vaporisation or sublimation. Accurate values for melting temperature are available for most metals, their boiling points being in some cases less certain,11 but the sublimation energy is the most useful quantity, this being the energy needed to secure complete atomisation of a given mass of metal. Division by the bulk co-ordination number gives the average bond strength. Figure 1.5 shows the periodic variation of sublimation heat for metals in Groups 1 to 14: there
Figure 1.5. Periodic variation of the heats of sublimation of metals of Groups 1 to 14 (see Figure 1.4 for meaning of points).

METALS AND ALLOYS |
7 |
are curious differences between this figure and figure 1.4, in that maximum bond strength now appears at Group 5 or 6 instead of at Group 8 or thereabouts. The effect may originate in the variable contributions that the energy changes accompanying electronic reorganisation make to the energetics of sublimation. There is clearly no uniquely reliable way of measuring the strength of bonds between metal atoms.
We may pause at this point to review in a qualitative manner those factors that influence the strength of intermetallic bonds. Clearly the number of valence electrons is of prime importance, but there are three other effects, not all equally obvious or apparent, that have to be noted. The Lanthanide Contraction has already been mentioned. This arises from the shape of the f -orbitals, which become filled between lanthanum and hafnium; they do not afford efficient shielding either of themselves or of other outer electrons from the nuclear charge, and hence they are all drawn towards the nucleus. This contraction, which is also shown to a minor extent as d-electron shells are filled, makes atomic sizes in the second and third Transition Series almost the same in corresponding Groups (Figures 1.3 and 1.4). Bond strengths however differ quite considerably, especially after Group 5 (Figure 1.5).
A second important effect is the stability of the half-filled d-shell. This is responsible for the unusual structure and chemistry of manganese, and for its low sublimation enthalpy (Figure 1.5) and melting temperature. The effect is also present, but less marked, in the second Transition Series, and is barely observable in the third; it is somehow anticipated by chromium and molybdenum in Group 5 (Figure 1.5), which have lower sublimation enthalpies than might otherwise have been expected.
The third factor is the most subtle and least well appreciated. In consequence of the Special Theory of Relativity, the mass m of a moving object increases with its speed v:
m = mo/√(1 − (v/c)2) |
(1.1) |
where mo is its rest mass and c the speed of light. For atoms with atomic number greater than about 50, the 1s electrons are sufficiently influenced by the nuclear mass that their speed becomes a substantial fraction of that of light (for mercury, Z = 80, v/c = 0.58) and their mass increases correspondingly.12−18 The size of the orbital contracts (by 23% in the case of mercury); and outer s shells also shrink in consequence, since orthogonality must be preserved. Electrons in p-orbitals are also affected, but d and f electrons less so, because their probability of being found near the nucleus is low. However, their effective potentials are more efficiently screened because of the relative contraction of the s and p shells; they therefore increase in energy and expand radially. The Schrodinger¨ equation is non-relativistic, and in effect assumes the speed of light to be infinite, and for heavier atoms the relativistic Dirac equation should be used.19 Although

8 |
CHAPTER 1 |
Dirac himself dismissed the idea that relativity could impinge on chemistry, he was in fact mistaken, and certainly all the third Transition Series and later elements are subject to its influence. The resulting orbital contraction is additional to the non-relativistic Lanthanide Contraction, and is partly responsible for the close correspondence in sizes between the second and third Series, already noted. It also accounts for the difference in colour and in chemistry between silver and gold, for the unusual structure and weak bonding in mercury, and for many other facets of the chemistry of the heavier elements traditionally associated with the stability of the 6s2 electron pair.1,12−18,20−22 Gold is the most electronegative metal, and forms salt-like compounds with very electropositive elements (e.g. Cs+Au−)
So far we have considered only those properties of metals that are attributable to the strength of the bonds between the atoms: however, towards the end of each Transition Series there are more valence electrons than can be accommodated in bonding orbitals, and those in excess are in effect localised on individual atoms. These also contribute importantly to the electronic properties of metals. All metals are good conductors of electricity, but some are better than others. There is little regularity in the variation of atomic conductance (i.e. specific conductance/atomic volume) across the Periodic Table; values are high in Groups 1 and 2, and exceptionally so in Group 11, but are very low for manganese and mercury, due no doubt to their unusual structures.10 Thermal conductance closely parallels electrical conductance, in line with the Wiedemann-Franz Law, which states that for all metals their ratio is a constant at a fixed temperature; its value is proportional to absolute temperature (the Wiedemann-Franz-Lorentz Law23).
Metals also show a range of magnetic properties.24 The magnetic susceptibility κ measures the ease of magnetisation:
I = κH |
(1.2) |
where I is the intensity of magnetisation and H the field strength. Paramagnetic substances have positive values of κ, and diamagnetic materials negative values. All metals of the Transition Series show weak, temperature-independent paramagnetism, except for the ferromagnetic iron, cobalt and nickel, which can be permanently magnetised below the Curie temperature and show the normal paramagnetism above it. The saturation moment of magnetisation (or the atomic magnetic moment) when expressed in Bohr magnetons gives the average number of unpaired electrons at zero Kelvin; this is another fixed point for explanation by theories of the metallic state. Manganese, technetium and palladium all have very high magnetic susceptibilities.
An interesting and potentially very useful property of the metallic state is superconductivity: the conductivity of a number of metals and alloys increases dramatically at very low temperatures, as the electrons pass through the rigid

METALS AND ALLOYS |
9 |
lattice of nuclei almost without obstruction. The phenomena is however of little relevance to catalysis.
1.1.2. Theories of the Metallic State10,25–30
It is no mere accident that the human race is designated as Homo sapiens, because it is the desire to know, and to understand the causes of things, that distinguishes us from other living creatures. The value of a good theory or explanation lies in its ability to correlate a wide range of observed phenomena by a simple model which is derived from the behaviour of the basic constituents of matter with the fewest possible assumptions. Theoretical descriptions of the metallic state rest on a knowledge of how the valence electrons behave; the correspondence between expectation and observation tests the precision of this knowledge and the methodology used to apply it.
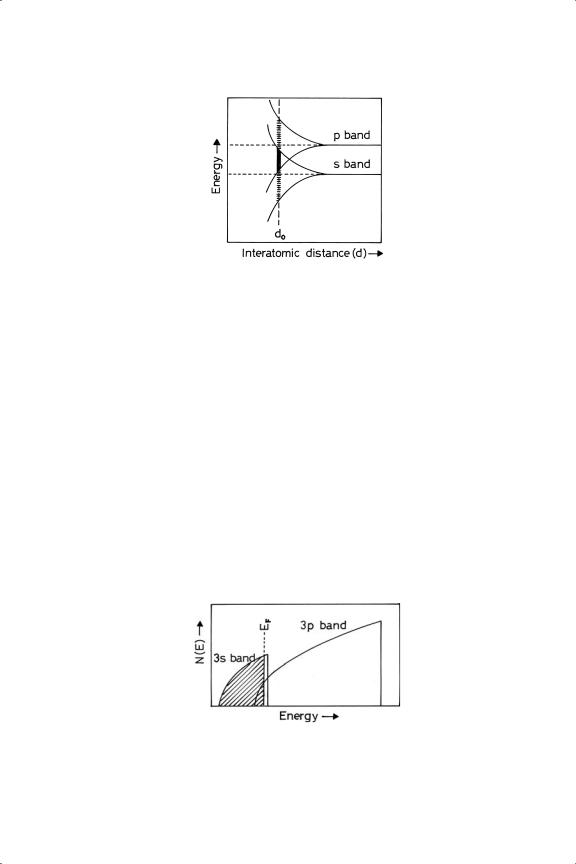
A firm foundation for the theory of metals only became possible with the advent of the Quantum Theory and the application of the Schrodinger¨ equation to electron waves: in particular the realisation, embodied in the Pauli Exclusion Principle, namely, that within a given system no more than two electrons can exist in the same energy state, was of fundamental importance. In a free atom in the ground state, electrons occupy definite energy levels corresponding to orbitals designated s, p, d, or f , according to the relevant quantum numbers. When however a number of atoms of the order of 1020 come together to form a metal crystal, their valence electrons cannot all continue to be in precise levels because by the Pauli Exclusion Principle no two electrons can have exactly the same values of all four quantum numbers; each is therefore compelled to take a microscopically different energy, but the energy difference between adjacent levels is however only about 10−40 J, and to all intents and purposes we may think of them occupying an energy band.23,31,32 The width of the band depends on the interatomic distance, as shown in Figure 1.6, and the number of levels within the band is determined by the number of atoms in the assembly. The inner electron levels still behave as such, because the interatomic spacing is too great for them to interact (Figure 1.6); for them each atom is an isolated system, which is why sharp Kα emission lines are obtained when transitions occur between K and L shells, and why the frequency of such lines is not affected by the state of chemical combination of the element. Bands may overlap to form hybrid bands as shown in Figure 1.6.
We now need to know how the probability of finding an electron of specified energy varies across the permitted band. In the first and simplest version of the Electron Band Theory, electrons were assumed to move in a field of uniform positive potential (i.e. ion cores were neglected), and mutual electrostatic repulsion was ignored. Application of the Schrodinger¨ equation and Fermi-Dirac statistics leads to the conclusion that a collection of N electrons at the absolute zero occupies the N/2 lowest levels, those at the maximum being said to be at the Fermi surface E F .
10 |
CHAPTER 1 |
Figure 1.6. Overlap of s and p bands as a function of interatomic distance: d0 shows the normal separation of atoms in the solid.
The number of energy states in a minuscule interval dE is termed the electron level density or density of states n(E) and this is proportional to E 1/2 (Figure 1.7). This highly simplified theory worked quite well for metals having only s and p electrons (sodium, magnesium, aluminium, tin), and provided the first reasonable interpretation of their electronic specific heats: it also led to a precise expression for the Wiedemann-Franz ratio.23,33
Extension of the Band Theory to the metals of the Transition Series required the introduction of ion cores into the argument. While for sodium the ion core is about 10% of the atomic volume, for Transition metals it is a much larger fraction, and cannot be ignored. Although in the case of an alkali metal the nature of the ion core is unambiguously defined, with Transition metals the core will not always have an inert gas configuration, and its structure has to be assumed before the potential field of the crystal can be defined. Moreover the location of the nuclei has to be precisely defined. It is a major weakness of the Band Theory that it does not address the directional nature of bonding between metal atoms
Figure 1.7. Electron level density diagram for magnesium based on simple band theory; the overlap of the 3s and 3 p bands allows electrical conduction.
