
- •ICU Protocols
- •Preface
- •Acknowledgments
- •Contents
- •Contributors
- •1: Airway Management
- •Suggested Reading
- •2: Acute Respiratory Failure
- •Suggested Reading
- •Suggested Reading
- •Website
- •4: Basic Mechanical Ventilation
- •Suggested Reading
- •Suggested Reading
- •Websites
- •Suggested Reading
- •Websites
- •7: Weaning
- •Suggested Reading
- •8: Massive Hemoptysis
- •Suggested Reading
- •9: Pulmonary Thromboembolism
- •Suggested Reading
- •Suggested Reading
- •Websites
- •11: Ventilator-Associated Pneumonia
- •Suggested Readings
- •12: Pleural Diseases
- •Suggested Reading
- •Websites
- •13: Sleep-Disordered Breathing
- •Suggested Reading
- •Websites
- •14: Oxygen Therapy
- •Suggested Reading
- •15: Pulse Oximetry and Capnography
- •Conclusion
- •Suggested Reading
- •Websites
- •16: Hemodynamic Monitoring
- •Suggested Reading
- •Websites
- •17: Echocardiography
- •Suggested Readings
- •Websites
- •Suggested Reading
- •Websites
- •19: Cardiorespiratory Arrest
- •Suggested Reading
- •Websites
- •20: Cardiogenic Shock
- •Suggested Reading
- •21: Acute Heart Failure
- •Suggested Reading
- •22: Cardiac Arrhythmias
- •Suggested Reading
- •Website
- •23: Acute Coronary Syndromes
- •Suggested Reading
- •Website
- •Suggested Reading
- •25: Aortic Dissection
- •Suggested Reading
- •26: Cerebrovascular Accident
- •Suggested Reading
- •Websites
- •27: Subarachnoid Hemorrhage
- •Suggested Reading
- •Websites
- •28: Status Epilepticus
- •Suggested Reading
- •29: Acute Flaccid Paralysis
- •Suggested Readings
- •30: Coma
- •Suggested Reading
- •Suggested Reading
- •Websites
- •32: Acute Febrile Encephalopathy
- •Suggested Reading
- •33: Sedation and Analgesia
- •Suggested Reading
- •Websites
- •34: Brain Death
- •Suggested Reading
- •Websites
- •35: Upper Gastrointestinal Bleeding
- •Suggested Reading
- •36: Lower Gastrointestinal Bleeding
- •Suggested Reading
- •37: Acute Diarrhea
- •Suggested Reading
- •38: Acute Abdominal Distension
- •Suggested Reading
- •39: Intra-abdominal Hypertension
- •Suggested Reading
- •Website
- •40: Acute Pancreatitis
- •Suggested Reading
- •Website
- •41: Acute Liver Failure
- •Suggested Reading
- •Suggested Reading
- •Websites
- •43: Nutrition Support
- •Suggested Reading
- •44: Acute Renal Failure
- •Suggested Reading
- •Websites
- •45: Renal Replacement Therapy
- •Suggested Reading
- •Website
- •46: Managing a Patient on Dialysis
- •Suggested Reading
- •Websites
- •47: Drug Dosing
- •Suggested Reading
- •Websites
- •48: General Measures of Infection Control
- •Suggested Reading
- •Websites
- •49: Antibiotic Stewardship
- •Suggested Reading
- •Website
- •50: Septic Shock
- •Suggested Reading
- •51: Severe Tropical Infections
- •Suggested Reading
- •Websites
- •52: New-Onset Fever
- •Suggested Reading
- •Websites
- •53: Fungal Infections
- •Suggested Reading
- •Suggested Reading
- •Website
- •55: Hyponatremia
- •Suggested Reading
- •56: Hypernatremia
- •Suggested Reading
- •57: Hypokalemia and Hyperkalemia
- •57.1 Hyperkalemia
- •Suggested Reading
- •Website
- •58: Arterial Blood Gases
- •Suggested Reading
- •Websites
- •59: Diabetic Emergencies
- •59.1 Hyperglycemic Emergencies
- •59.2 Hypoglycemia
- •Suggested Reading
- •60: Glycemic Control in the ICU
- •Suggested Reading
- •61: Transfusion Practices and Complications
- •Suggested Reading
- •Websites
- •Suggested Reading
- •Website
- •63: Onco-emergencies
- •63.1 Hypercalcemia
- •63.2 ECG Changes in Hypercalcemia
- •63.3 Superior Vena Cava Syndrome
- •63.4 Malignant Spinal Cord Compression
- •Suggested Reading
- •64: General Management of Trauma
- •Suggested Reading
- •65: Severe Head and Spinal Cord Injury
- •Suggested Reading
- •Websites
- •66: Torso Trauma
- •Suggested Reading
- •Websites
- •67: Burn Management
- •Suggested Reading
- •68: General Poisoning Management
- •Suggested Reading
- •69: Syndromic Approach to Poisoning
- •Suggested Reading
- •Websites
- •70: Drug Abuse
- •Suggested Reading
- •71: Snakebite
- •Suggested Reading
- •72: Heat Stroke and Hypothermia
- •72.1 Heat Stroke
- •72.2 Hypothermia
- •Suggested Reading
- •73: Jaundice in Pregnancy
- •Suggested Reading
- •Suggested Reading
- •75: Severe Preeclampsia
- •Suggested Reading
- •76: General Issues in Perioperative Care
- •Suggested Reading
- •Web Site
- •77.1 Cardiac Surgery
- •77.2 Thoracic Surgery
- •77.3 Neurosurgery
- •Suggested Reading
- •78: Initial Assessment and Resuscitation
- •Suggested Reading
- •79: Comprehensive ICU Care
- •Suggested Reading
- •Website
- •80: Quality Control
- •Suggested Reading
- •Websites
- •81: Ethical Principles in End-of-Life Care
- •Suggested Reading
- •82: ICU Organization and Training
- •Suggested Reading
- •Website
- •83: Transportation of Critically Ill Patients
- •83.1 Intrahospital Transport
- •83.2 Interhospital Transport
- •Suggested Reading
- •84: Scoring Systems
- •Suggested Reading
- •Websites
- •85: Mechanical Ventilation
- •Suggested Reading
- •86: Acute Severe Asthma
- •Suggested Reading
- •87: Status Epilepticus
- •Suggested Reading
- •88: Severe Sepsis and Septic Shock
- •Suggested Reading
- •89: Acute Intracranial Hypertension
- •Suggested Reading
- •90: Multiorgan Failure
- •90.1 Concurrent Management of Hepatic Dysfunction
- •Suggested Readings
- •91: Central Line Placement
- •Suggested Reading
- •92: Arterial Catheterization
- •Suggested Reading
- •93: Pulmonary Artery Catheterization
- •Suggested Reading
- •Website
- •Suggested Reading
- •95: Temporary Pacemaker Insertion
- •Suggested Reading
- •96: Percutaneous Tracheostomy
- •Suggested Reading
- •97: Thoracentesis
- •Suggested Reading
- •98: Chest Tube Placement
- •Suggested Reading
- •99: Pericardiocentesis
- •Suggested Reading
- •100: Lumbar Puncture
- •Suggested Reading
- •Website
- •101: Intra-aortic Balloon Pump
- •Suggested Reading
- •Appendices
- •Appendix A
- •Appendix B
- •Common ICU Formulae
- •Appendix C
- •Appendix D: Syllabus for ICU Training
- •Index

142 |
R. Pandit and J.V. Divatia |
|
|
•Signs of tamponade
–Right atrium—early systolic collapse
–Right ventricle—early diastolic collapse
–IVC diameter and collapsibility—dilated and/or fixed.
Suggested Readings
1.Beaulieu Y, Marik PE. Bed side ultrasonography in ICU part-I. Chest. 2005;128:881–95.
2.Beaulieu Y, Marik PE. Bed side ultrasonography in ICU part-II. Chest. 2005;128:1766–81.
A comprehensive review on the subject.
3.Brennan JM, Blair JE, Goonewardena S, Ronan A, Shah D, Vasaiwala S, Kirkpatrick JN, Spencer KT. Reappraisal of the use of IVC for estimating RAP. J Am Soc Echocardiogr. 2007;20(7):857–61.
The IVC size cutoff with optimum predictive use for RAP above or below 10 mmHg was 2.0 cm (sensitivity 73% and specificity 85%) and the optimal IVC collapsibility cutoff was 40% (sensitivity 73% and specificity 84%). Traditional classification of RAP into 5 mmHg ranges based on IVC size and collapsibility performed poorly (43% accurate) and a new classification scheme is proposed.
4.Jones R, Blaivas M. The handbook of ultrasound in trauma and critical illness. Ohio: ACEP; 2003.
Websites
1.www.asecho.org
Homepage for American Society of Echocardiography
2.www.niccer.org.au Website of Nepean Institute
3.www.criticalecho.com/content/icu-echo-tutorials An excellent site from CMC Vellore, India
4.www.sonoguide.com/cardiac.html
Excellent virtual illustration of bedside echo windows
Fluid Therapy, Vasopressors, and |
18 |
Inotropes |
Jigeeshu V. Divatia and Parvez Ali Khan
A 55-year-old male patient presented with acute respiratory distress. His heart rate was 140/min, BP was 70/40 mmHg, and respiratory rate was 34 breaths/min. Core temperature was 34.4°C. He was oliguric, and the abdomen was tender, firm, and distended. He was intubated and mechanically ventilated. A central venous catheter was inserted, and the central venous pressure (CVP) was 18 mmHg. He remained tachycardic and hypotensive.
Fluids (crystalloid or colloid), vasopressors, and inotropes are common interventions in managing unstable patients in the ICU. These interventions should be used judiciously to maintain perfusion to vital organs while definitive therapy is being instituted. On the other hand, if they are used injudiciously, these interventions may be potentially harmful.
Step 1: Start fluids for resuscitation
•In patients with new-onset hypotension, tachycardia, unexplained oliguria, or other evidence of hypoperfusion such as increased lactate or base deficit, consider a fluid challenge, if this seems to be of low risk clinically, for example, patients with no evidence of overt heart failure.
•Administer 500–1,000 mL crystalloid or 250–500 mL colloid over 30 min. In patients with sepsis, crystalloids are the preferred initial resuscitation fluid.
J.V. Divatia, M.D., F.I.S.C.C.M. (*)
Department of Anaesthesiology, Critical Care and Pain, Tata Memorial Hospital, Mumbai, India e-mail: jdivatia@yahoo.com
P.A. Khan, M.D.
Department of Anaesthesiology, Critical Care and Pain, Tata Memorial Hospital,
Mumbai, India
R. Chawla and S. Todi (eds.), ICU Protocols: A stepwise approach, |
143 |
DOI 10.1007/978-81-322-0535-7_18, © Springer India 2012 |
|
144 |
J.V. Divatia and P.A. Khan |
|
|
•Fluid challenge in the ICU should be protocolized with clear direction about the various components of fluid challenge—type of fluid, rate of administration, clinical and pressure end points, and safety limits (see Chap. 16).
•Target CVP should be 8–12 mmHg in spontaneously breathing patients and 12–15 mmHg in mechanical ventilated patients or those with preexisting decreased ventricular compliance, for example, in hypertensives.
•These parameters need to be individualized depending on patients’ clinical status, comorbidity, and underlying pathology.
•Careful monitoring of patients clinically and hemodynamically is mandatory during fluid challenge to assess response as well as evidence of fluid overload.
Step 2: Select fluid for resuscitation
•There is no ideal fluid for resuscitation. Outcomes are similar using either 0.9% normal saline (crystalloid) or 4% albumin (colloid) for fluid resuscitation. However, hemodynamic goals tend to be achieved faster with colloid resuscitation.
•Crystalloids are cheaper but infusion of large volumes of normal saline can cause hyperchloremic acidosis, while colloids may cause coagulopathy, renal dysfunction and anaphylactoid reactions.
•The total volume of HES 130/0.4 should be restricted to 50 mL/Kg/day. The older generation of starches (HES 200/0.5) should be avoided, as they have been shown to be associated with higher incidence of renal failure and coagulopathy, and if at all used, the volume should be restricted to 33 mL/Kg/day. The new generation of starches (HES 130/0.4) appear to have a lower incidence of coagulopathy.
•All hyperoncotic colloids (including 10% HES and 20% albumin) can precipitate renal dysfunction. They should always be combined with crystalloids.
•Most synthetic colloids are prepared in solution with normal saline. Hence, large volumes of such colloids can also cause hyperchloremic acidosis with resultant coagulopathy.
•Crystalloids (lactated Ringer’s solution) and colloids in balanced solution do not cause hyperchloremic acidosis and may be preferable to normal saline for large volume resuscitation.
Step 3: Assess response
•Response to a fluid challenge should be assessed clinically by features of increased perfusion like improved sensorium, increased sense of well-being, and increased urine output.
•This should also be assessed by improvement of hemodynamic parameters such as decreased tachycardia, improved blood pressure, and improved CVP or wedge pressure.
•Increasingly, it has been realized that static measures of preload such as CVP or pulmonary artery occlusion pressure (PAOP) do not adequately reflect need for fluid challenges. There are two principal reasons for this realization:
–As per Frank–Starling law, preload (CVP or PAOP) is related to stroke volume or cardiac output in a curvilinear manner; that is, rise in preload will result in
18 Fluid Therapy, Vasopressors, and Inotropes |
145 |
|
|
increasing stroke volume in the steep part of the curve till the flat part is reached when increasing preload will not lead to further increase in stroke volume. In a given patient, it is difficult to predict the position of a preload measure on this curve by static values as it is dependent on ventricular compliance which is variable.
–Left ventricular compliance varies among patients and may vary at different times in the same patient, so the pressure–volume curves are variable; thus, a stiff ventricle (hypertrophy) will lead to a decrease in ventricular compliance with shift of pressure–volume curves of the ventricle to the left. It means high pressure values (CVP or PAOP) for the same or low ventricular volume and a more compliant (dilated) ventricle will shift this curve to the right; thus, a low pressure (CVP or PAOP) reading may indicate a high ventricular volume. Thus, it is difficult to predict ventricular volume by a given pressure index (CVP or PAOP).
•Dynamic indices such as pulse pressure variation (PPV), systolic pressure variation (SPV) or stroke volume variation (SVV), echocardiographic vena cava diameter, or esophageal Doppler aortic blood flow changes during controlled positive-pressure ventilation or during passive leg raising in spontaneously breathing patients are more representative for predicting fluid responsiveness (see Chap. 16).
•When the risk of fluid challenge is not trivial, for example, in patients with compromised lung or cardiac function, consider using a dynamic predictor to guide fluid boluses.
•Since the hemodynamic state changes rapidly, reassessment of hemodynamics should be done frequently.
Step 4: Select inotrope or vasopressors
•Despite adequate volume replacement, if the patient is hypotensive and perfusion of vital organs is jeopardized, vasoactive agents may be administered to improve cardiac output and blood pressure.
•It is useful to understand the receptors through which adrenergic agents exert their effect.
•Three broad groups of agents may be identified:
1.Predominant b-agonists (dobutamine, dopexamine, isoprenaline)
2.Predominant a-agonists (phenylephrine)
3.Those with mixed b- and a-effects (adrenaline and noradrenaline).
•In general, when the heart is failing, and the peripheral vascular resistance is normal, an agent with predominant inotropic effect (especially a b-1 selective agent) would be a good choice.
•If there is vasodilatation, a vasoconstrictor with predominant a-agonist activity is appropriate.
•Familiarize with doses and effects of commonly used inotropes and vasopressors.
•Consider practical aspects of vasopressor infusion:
– Infuse through large veins preferably central veins.
– Use multi-lumen catheters and use dedicated lumen for vasopressor infusion.
– No other drug bolus or infusion should be given through the same lumen.
146 |
J.V. Divatia and P.A. Khan |
|
|
–Use infusion or syringe pumps or other infusion controllers.
–Invasive arterial pressure should be measured.
–Dobutamine and other inodilators may be given through peripheral line.
•Volume deficit should always be corrected as much as possible before resorting to vasopressors which would lead to a false sense of security by increasing blood pressure while underlying hypovolemia and resultant low perfusion will lead to subsequent organ dysfunction.
Step 5: Titrate inotropes and vasopressors
•All inotropes and vasopressors should be titrated so that tissue perfusion is restored with the lowest dose of drug and to the desired end points with minimal or no side effects:
–Titrate to clinical improvements in heart rate (HR) and mean arterial pressure (MAP).
–Titrate inotropes to desired cardiac output.
•Do not aim for supranormal cardiac output:
–Titrate vasopressors to MAP of 65–70 mmHg.
–In patients with long-standing hypertension, renal failure, recent cerebral infarct, and increased intra-abdominal pressure, a higher MAP may be desirable.
–In trauma with active bleeding, a lower MAP till bleeding source is controlled is advisable.
–Aiming for higher MAP than desired may result in unnecessary vasoconstriction.
–Titrate to achieve adequacy of organ perfusion
•Urine output more than 0.5 mL/Kg/h
•ScvO2 more than 70%
•Reduction in lactate levels over time (e.g., 20% over 2 h)
•Watch for side effects: tachycardia, arrhythmias, cardiac ischemia.
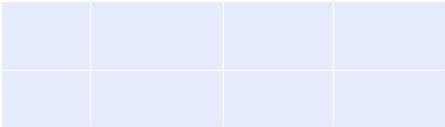
Step 6: Customize use of inotropes and vasopressors (Tables 18.1 and 18.2)
•Choice of inotropes and vasopressors may vary depending on clinical situation.
•Levosimendan
Table 18.1 Agents used in myocardial infarction and cardiogenic shock
Clinical picture |
Low cardiac index (CI) |
Low CI < 2.2 L/min/ |
Low CI < 2.2 L/min/ |
|
but systolic blood pressure |
M2, SBP < 90 mmHg, |
M2, SBP < 90 mmHg, |
|
(SBP) > 100 mmHg, high |
high LV filling |
high right atrium and |
|
left ventricular (LV) filling |
pressures |
right ventricular |
|
pressures |
|
diastolic pressures |
Choice of |
Dobutamine/milrinone |
Dopamine/ |
Volume replacement/ |
inotropic agents |
|
noradrenaline/ |
dobutamine and |
|
|
intra-aortic balloon |
noradrenaline/IABP |
|
|
pump (IABP) |
|
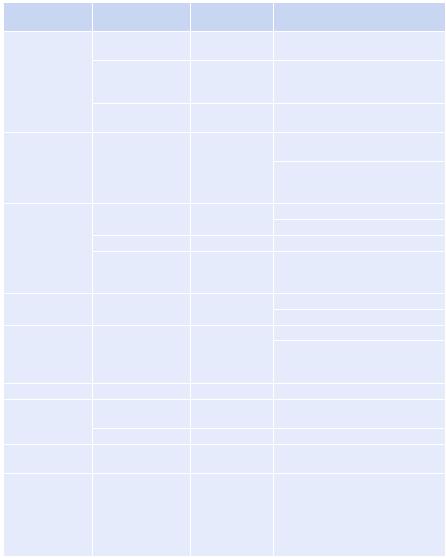
18 Fluid Therapy, Vasopressors, and Inotropes |
147 |
||
|
|||
Table 18.2 Doses and effects of commonly used inotropes and vasopressors |
|||
|
Dose/range |
Predominant |
|
Drug |
(mg/Kg/min) |
receptor |
Effects |
Adrenaline |
0.01–0.02 |
b-2 |
Lowered systemic vascular |
|
|
|
resistance (SVR), BP |
|
0.03–0.20 |
b-1 |
Increased contractility, HR, cardiac |
|
|
|
output (CO), lactic acidosis, |
|
|
|
hyperglycemia |
|
0.20–0.30 |
Alpha |
Increased SVR, BP, impaired |
|
|
|
splanchnic perfusion |
Noradrenaline |
0.01–0.40 |
a-1, a-2, b-2 |
Raised SVR, BP, possible reflex |
|
|
|
fall in HR, possible fall in CO |
|
|
|
In fluid-resuscitated patients, |
|
|
|
improved renal and splanchnic |
|
|
|
blood flow |
Dopamine |
0.01–3.00 |
Dopaminergic |
Renal and splanchnic |
|
|
|
Vasodilatation |
|
3.0–7.0 |
b-1 |
Increased contractility, HR, CO |
|
>7.0 |
Alpha 1 |
Increased SVR, BP, variable effects |
|
|
|
on splanchnic circulation and |
|
|
|
gastric mucosal flow |
Dobutamine |
3.0–20.0 |
b-1 (plus some |
Increased contractility, decreased |
|
|
b-2, a) |
SVR, increased CO, increased HR |
Dopexamine |
0.5–6.0 |
b-2 (plus some |
Increased contractility, decreased |
|
|
b-1, a and |
SVR, increased CO, increased HR, |
|
|
dopaminergic) |
increased renal and splanchnic |
|
|
|
blood flow at low dose |
Isoprenaline |
0.01–0.03 |
b-1, b-2 |
Decreased SVR, increased HR |
Milrinone bolus |
50 mg/Kg |
|
Increased cAMP, increased |
|
|
|
contractility, coronary blood flow |
|
0.35–0.75 |
|
Decreased SVR, PVR, arrhythmias |
Phenylephrine |
0.1–3.0 |
Alpha 1 |
Prolonged action potential, |
|
|
|
Increased SVR, inotropy |
Levosimendan |
6–12 mg/Kg |
Calcium |
Increased sensitivity of actinomy- |
|
loading dose over |
sensitizer |
cin to calcium. Increased myocar- |
|
10 min followed by |
|
dial contractility. |
|
0.05–0.2 mg/Kg/ |
|
|
|
min as a continuous |
|
|
|
infusion |
|
|
–It is a myofilament calcium sensitizer. It increases myocardial contractility without increasing myocardial ATP consumption, thereby improving contraction at low energy cost.
–It causes normal or improved diastolic relaxation and vasodilatation.
–It has been studied in acute decompensated heart failure, during and after cardiac surgery, and postmyocardial infarction.
148 |
J.V. Divatia and P.A. Khan |
|
|
•Digitalis glycosides
–The digitalis glycosides have long been used as inotropic agents.
–However, today their role in the treatment of acute heart failure or cardiogenic shock is limited to control of the ventricular rate response in fast atrial fibrillation.
–The onset of action of effects of digoxin takes 90 min after an intravenous loading dose, and peak effect occurs at 2–6 h.
–The effects of digoxin are modest and unpredictable, and it has a narrow therapeutic index.
•Agents used in septic shock
The Surviving Sepsis Campaign makes the following evidence-based recommendations in patients with sepsis:
–Vasopressors
•Recommend to maintain MAP ³ 65 mmHg.
•Recommend noradrenaline centrally administered as the initial vasopressors of choice.
•Suggest that dopamine, adrenaline, phenylephrine, or vasopressin should not be administered as the initial vasopressor in septic shock.
•Vasopressin at dose of 0.03 unit/min may be subsequently added to noradrenaline with anticipation of an effect equivalent to noradrenaline alone.
•Use adrenaline as the first alternative agent in septic shock when blood pressure is poorly responsive to noradrenaline
•Recommend not to use low-dose dopamine for renal protection.
•Recommend to insert an arterial catheter in patients requiring vasopressors, as soon as practical.
–Inotropic therapy
•Recommend the use of dobutamine in patients with myocardial dysfunction as supported by elevated cardiac filling pressures and low cardiac output.
•Do not increase cardiac index to predetermined supranormal levels.
Step 7: Understand limitations of vasopressor and inotrope therapy
•All inotropic and vasopressor drugs may increase myocardial oxygen demand.
•Increasing blood pressure by use of vasopressors does not lead to increased perfusion all the time; in certain circumstances like hypovolemia, it might lead to decreased flow to end organs.
•Tachycardia may occur, especially in volume-depleted patients.
•Arrhythmias.
•Catecholamines also have significant neurohumoral and metabolic effects, which might be deleterious, for example, hyperglycemia and hyperlactatemia induced by adrenaline and suppression of prolactin by dopamine.
Step 8: Wean inotropes and vasopressors
•All attempts should be made to treat underlying cause of low perfusion state whenever feasible and vasopressors should be weaned off at the earliest.
•If necessary, additional fluid challenges may be used judiciously in order to wean off vasopressors.
