
- •ICU Protocols
- •Preface
- •Acknowledgments
- •Contents
- •Contributors
- •1: Airway Management
- •Suggested Reading
- •2: Acute Respiratory Failure
- •Suggested Reading
- •Suggested Reading
- •Website
- •4: Basic Mechanical Ventilation
- •Suggested Reading
- •Suggested Reading
- •Websites
- •Suggested Reading
- •Websites
- •7: Weaning
- •Suggested Reading
- •8: Massive Hemoptysis
- •Suggested Reading
- •9: Pulmonary Thromboembolism
- •Suggested Reading
- •Suggested Reading
- •Websites
- •11: Ventilator-Associated Pneumonia
- •Suggested Readings
- •12: Pleural Diseases
- •Suggested Reading
- •Websites
- •13: Sleep-Disordered Breathing
- •Suggested Reading
- •Websites
- •14: Oxygen Therapy
- •Suggested Reading
- •15: Pulse Oximetry and Capnography
- •Conclusion
- •Suggested Reading
- •Websites
- •16: Hemodynamic Monitoring
- •Suggested Reading
- •Websites
- •17: Echocardiography
- •Suggested Readings
- •Websites
- •Suggested Reading
- •Websites
- •19: Cardiorespiratory Arrest
- •Suggested Reading
- •Websites
- •20: Cardiogenic Shock
- •Suggested Reading
- •21: Acute Heart Failure
- •Suggested Reading
- •22: Cardiac Arrhythmias
- •Suggested Reading
- •Website
- •23: Acute Coronary Syndromes
- •Suggested Reading
- •Website
- •Suggested Reading
- •25: Aortic Dissection
- •Suggested Reading
- •26: Cerebrovascular Accident
- •Suggested Reading
- •Websites
- •27: Subarachnoid Hemorrhage
- •Suggested Reading
- •Websites
- •28: Status Epilepticus
- •Suggested Reading
- •29: Acute Flaccid Paralysis
- •Suggested Readings
- •30: Coma
- •Suggested Reading
- •Suggested Reading
- •Websites
- •32: Acute Febrile Encephalopathy
- •Suggested Reading
- •33: Sedation and Analgesia
- •Suggested Reading
- •Websites
- •34: Brain Death
- •Suggested Reading
- •Websites
- •35: Upper Gastrointestinal Bleeding
- •Suggested Reading
- •36: Lower Gastrointestinal Bleeding
- •Suggested Reading
- •37: Acute Diarrhea
- •Suggested Reading
- •38: Acute Abdominal Distension
- •Suggested Reading
- •39: Intra-abdominal Hypertension
- •Suggested Reading
- •Website
- •40: Acute Pancreatitis
- •Suggested Reading
- •Website
- •41: Acute Liver Failure
- •Suggested Reading
- •Suggested Reading
- •Websites
- •43: Nutrition Support
- •Suggested Reading
- •44: Acute Renal Failure
- •Suggested Reading
- •Websites
- •45: Renal Replacement Therapy
- •Suggested Reading
- •Website
- •46: Managing a Patient on Dialysis
- •Suggested Reading
- •Websites
- •47: Drug Dosing
- •Suggested Reading
- •Websites
- •48: General Measures of Infection Control
- •Suggested Reading
- •Websites
- •49: Antibiotic Stewardship
- •Suggested Reading
- •Website
- •50: Septic Shock
- •Suggested Reading
- •51: Severe Tropical Infections
- •Suggested Reading
- •Websites
- •52: New-Onset Fever
- •Suggested Reading
- •Websites
- •53: Fungal Infections
- •Suggested Reading
- •Suggested Reading
- •Website
- •55: Hyponatremia
- •Suggested Reading
- •56: Hypernatremia
- •Suggested Reading
- •57: Hypokalemia and Hyperkalemia
- •57.1 Hyperkalemia
- •Suggested Reading
- •Website
- •58: Arterial Blood Gases
- •Suggested Reading
- •Websites
- •59: Diabetic Emergencies
- •59.1 Hyperglycemic Emergencies
- •59.2 Hypoglycemia
- •Suggested Reading
- •60: Glycemic Control in the ICU
- •Suggested Reading
- •61: Transfusion Practices and Complications
- •Suggested Reading
- •Websites
- •Suggested Reading
- •Website
- •63: Onco-emergencies
- •63.1 Hypercalcemia
- •63.2 ECG Changes in Hypercalcemia
- •63.3 Superior Vena Cava Syndrome
- •63.4 Malignant Spinal Cord Compression
- •Suggested Reading
- •64: General Management of Trauma
- •Suggested Reading
- •65: Severe Head and Spinal Cord Injury
- •Suggested Reading
- •Websites
- •66: Torso Trauma
- •Suggested Reading
- •Websites
- •67: Burn Management
- •Suggested Reading
- •68: General Poisoning Management
- •Suggested Reading
- •69: Syndromic Approach to Poisoning
- •Suggested Reading
- •Websites
- •70: Drug Abuse
- •Suggested Reading
- •71: Snakebite
- •Suggested Reading
- •72: Heat Stroke and Hypothermia
- •72.1 Heat Stroke
- •72.2 Hypothermia
- •Suggested Reading
- •73: Jaundice in Pregnancy
- •Suggested Reading
- •Suggested Reading
- •75: Severe Preeclampsia
- •Suggested Reading
- •76: General Issues in Perioperative Care
- •Suggested Reading
- •Web Site
- •77.1 Cardiac Surgery
- •77.2 Thoracic Surgery
- •77.3 Neurosurgery
- •Suggested Reading
- •78: Initial Assessment and Resuscitation
- •Suggested Reading
- •79: Comprehensive ICU Care
- •Suggested Reading
- •Website
- •80: Quality Control
- •Suggested Reading
- •Websites
- •81: Ethical Principles in End-of-Life Care
- •Suggested Reading
- •82: ICU Organization and Training
- •Suggested Reading
- •Website
- •83: Transportation of Critically Ill Patients
- •83.1 Intrahospital Transport
- •83.2 Interhospital Transport
- •Suggested Reading
- •84: Scoring Systems
- •Suggested Reading
- •Websites
- •85: Mechanical Ventilation
- •Suggested Reading
- •86: Acute Severe Asthma
- •Suggested Reading
- •87: Status Epilepticus
- •Suggested Reading
- •88: Severe Sepsis and Septic Shock
- •Suggested Reading
- •89: Acute Intracranial Hypertension
- •Suggested Reading
- •90: Multiorgan Failure
- •90.1 Concurrent Management of Hepatic Dysfunction
- •Suggested Readings
- •91: Central Line Placement
- •Suggested Reading
- •92: Arterial Catheterization
- •Suggested Reading
- •93: Pulmonary Artery Catheterization
- •Suggested Reading
- •Website
- •Suggested Reading
- •95: Temporary Pacemaker Insertion
- •Suggested Reading
- •96: Percutaneous Tracheostomy
- •Suggested Reading
- •97: Thoracentesis
- •Suggested Reading
- •98: Chest Tube Placement
- •Suggested Reading
- •99: Pericardiocentesis
- •Suggested Reading
- •100: Lumbar Puncture
- •Suggested Reading
- •Website
- •101: Intra-aortic Balloon Pump
- •Suggested Reading
- •Appendices
- •Appendix A
- •Appendix B
- •Common ICU Formulae
- •Appendix C
- •Appendix D: Syllabus for ICU Training
- •Index
Brain Death |
34 |
|
|
Babu K. Abraham and Nagarajan Ramakrishnan |
|
A 25-year-old male motorbike rider was brought to the intensive care unit (ICU) following a head-on collision with a car while traveling on a motorway. On arrival, he was found to have blood oozing out of his external auditory meatus and nostrils. Neurological assessment revealed Glasgow coma scale (GCS) of 2T, pupils equal, 3 mm bilaterally, and not reacting to light. Family and colleagues questioned whether he was “brain dead.”
The role of the ICU physician in these situations is to proceed systematically and according to the institutional protocol to diagnose and confirm brain death, counsel family, and support the patient for potential organ donation if indicated.
Step 1: Establish the underlying cause
•The potential cause may be obvious in some cases.
•This may not be clear in every situation, and if there is any doubt about the cause, be cautious in diagnosing brain death.
•Investigations such as brain imaging (CT scan and MRI) and cerebrospinal fluid (CSF) analysis may be useful in evaluation of etiology but is not confirmative of brain death.
•A search for confounders (see below) should be made systematically.
Step 2: Look for confounders before proceeding for brain death verification
•Complicating medical conditions that may mimic brainstem dysfunction (severe electrolyte abnormalities, hypoglycemia, and acid–base abnormalities).
•Severe hypothermia (core temperature of £32°C).
B.K. Abraham, M.D., M.R.C.P. (*) • N. Ramakrishnan, A.B.(I.M.), F.A.C.P. Department of Critical Care Medicine, Apollo Hospitals, Chennai, India e-mail: abrahambk@gmail.com
R. Chawla and S. Todi (eds.), ICU Protocols: A stepwise approach, |
275 |
DOI 10.1007/978-81-322-0535-7_34, © Springer India 2012 |
|
276 |
B.K. Abraham and N. Ramakrishnan |
|
|
•Severe hypotension (systolic blood pressure <100 mmHg).
•Any evidence of drug intoxication including alcohol, poisoning, or recent use of sedation or neuromuscular blocking agents.
•If any of the confounders are present, they need to be corrected, and in case of suspected poisoning, an extended observation period is needed before proceeding to further test.
•In circumstances, where these cannot be done, confirmatory test (see below) for brain death is needed.
Step 3: Identify brain death by complete neurological examination
This consists of three essential documentations:
1.Documentation of coma
•Absence of motor response to a standardized painful stimulus (pressing on supraorbital nerve, temporomandibular joints, or nail bed of a finger).
•Beware of local spinal reflexes causing spontaneous or stimulus-related motor movements.
2.Documentation of the absence of brainstem reflexes
•As brain death occurs, reflexes are lost in a rostral-to-caudal direction with the reflexes in medulla oblongata being the last to cease.
•Absent pupillary reflex—the pupils will be round or oval, with midposition dilatation (4–9 mm) and no reaction to bright light.
•Oculocephalic movements (doll’s eye reflex) will be absent. Ensure integrity of the cervical spine. This test should be done by rapid turning of the head horizontally and vertically and looking for eye movements. There should be no movement of the eyes to the opposite side in brainstem dysfunction. This test may be difficult to interpret at times.
•Oculovestibular reflex—cold calorie test will show the absence of provoked tonic eye movement toward the side of cold stimulus. This test is performed by irrigating the tympanum with 50 mL ice-cold water with the head tilted to 30°. Presence of clotted blood or cerumen in the external auditory canal can diminish this response even in the absence of brain death.
•Corneal reflex will be absent. This is checked by instilling a few drops of sterile saline over cornea.
•Cough reflex will be absent. This is best tested by passing a suction catheter through the endotracheal tube.
3.Documentation of apnea (apnea test)
•This test is carried out only after documenting the absence of brainstem reflexes and is performed to stimulate the respiratory center, which is in the medulla oblongata.
•The patient is first preoxygenated with 100% oxygen for 15 min, and an arterial blood gas analysis (ABG) is obtained.
•The patient is then disconnected from mechanical ventilation and continued to oxygenate through a catheter placed in the trachea with oxygen flow at 6–10 L/min. Alternatives include using a T-piece system with oxygen flow at
34 Brain Death |
277 |
|
|
12 L/min or using continuous positive airway pressure (CPAP) at 10 cm H2O with FiO2 titrated to keep oxygen saturation above 95%.
•PaCO2 is allowed to climb (usually at a rate of 3 mmHg/min). The threshold for maximal stimulation of the respiratory center is thought to be PaCO2 of 60 mmHg or a PaCO2 of 20 mmHg above the normal baseline value.
•ABG is repeated within about 8–10 min of disconnection from the mechanical ventilation, and the increase in the PaCO2 is documented.
•Visual observation is the standard method for detecting respiratory movement. About 8–10 min with no observable respiratory effort is a standard observation period. During this period of observation, if the subject does not
have spontaneous respiration and arterial PCO2 is 60 mmHg (or 20 mmHg increase over the baseline arterial PCO2), the apnea test result is positive (i.e., supports the clinical diagnosis of brain death).
•This test should not be performed or should be terminated if the patient becomes hemodynamically unstable or hypoxemic.
•If the test is inconclusive but the patient is hemodynamically stable during the procedure, it may be repeated for a longer period (10–15 min) after the patient is again adequately preoxygenated.
Step 4: Perform confirmatory tests
These tests are optional in adults but recommended in children younger than 1 year. In certain countries, these tests are required by law to confirm brain death:
•Cerebral angiography (conventional or CT)
–Angiography of both the anterior and posterior circulations has to be carried out. The absence of contrast flow into the intracerebral portions of the carotid and vertebral arteries at the level of their entry into the skull is taken as a sign of brain death.
–External carotid circulation should be patent.
–Delayed filling of the superior longitudinal sinus may be seen.
–Limitation of the test is the need for transporting the patient.
•Electroencephalography (EEG)
–A complete absence of electroencephalographic activity to intense somatosensory and audiovisual stimulation is taken as a sign of brain death.
–It is important to ensure that EEG recordings are done in a standardized manner.
–Limitations are presence of artifacts leading to an EEG pattern for falsenegative brain death diagnosis.
•Transcranial Doppler (TCD) ultrasound
–The TCD abnormalities that indicate brain death are a lack of diastolic or reverberating flow and the documentation of small systolic peaks in early systole.
–Complete absence of flow in the TCD may not be reliable owing to inadequate transtemporal windows.
–Limitation is the need for expertise and appropriate interpretation of the flow pattern.
278 |
B.K. Abraham and N. Ramakrishnan |
|
|
•Cerebral scintigraphy
–Demonstration of absent cerebral blood flow using radiolabeled (99mTclabeled) hexamethylpropyleneamine oxime (HMPAO), followed by single photon emission computerized tomography (SPECT) scanning, provides a confirmatory test in the diagnosis of brain death.
–The absence of isotope uptake (“hollow skull phenomenon”) indicates no brain perfusion and supports the diagnosis of brain death.
–Limitation is lack of availability and need for transportation.
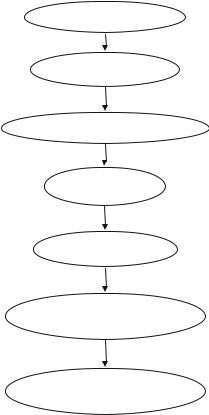
Step 5: Follow institutional protocol for certifying brain death (Fig. 34.1)
•Institutes should have a predefined protocol as regards to certifying authority and frequency of examination.
•Most places would mandate two sets of brain death examinations, the second typically done a minimum of 6 h after the first, to declare brain death in adults.
•In neonates and pediatrics, this period of observation is usually longer varying from 12 to 48 h.
•There is no consensus about how long a patient needs to be observed before being certain that the brain functions have ceased irreversibly.
Fig. 34.1 Brain death
management |
Identify cause of coma |
|
Exclude confounders
Perform brainstem reflex tests
Apnea test
Confirmatory tests
Follow institution protocol when informing family
Manage patient if a potential organ donor
34 Brain Death |
279 |
|
|
Step 6: Inform the family
•As soon as it is suspected that the patient is nearing brain death, the clinician should keep family members informed of the clinical status of the patient and maintain the patient in a hemodynamically stable state if he/she is a potential organ donor.
•As declaring brain death is a sensitive issue, the senior member of the team, ideally the primary consultant, should inform the next of kin after the clinical criteria for brain death have been confirmed.
•He or she then can be approached for organ donation as per the regulations of the state law.
•If the next of kin declines to donate organs, then it is good medical judgment to discontinue the mechanical ventilation (Fig. 34.1).
Steps in Managing a Brain-Dead Patient
Step 1: Monitor and manage hemodynamics
•Adequate monitoring is essential for managing a potential organ donor.
•If not already placed, an arterial line, a central venous line, and a urinary catheter will be useful.
•These will help in assessing resuscitation and monitoring the hemodynamic status closely.
•Adequate volume status should be maintained.
Step 2: Replace hormones
•Brain death is associated with a panhypopituitary state that can lead to refractory hypotension. Hormone replacement therapy not only helps in correcting this but also decreases cardiovascular instability and improves organ protection and graft function.
•The common hormones replaced are the following:
•Levothyroxine
–Thyroxine (T4) is initially given at 20 mcg IV bolus. T4 has been shown to be more beneficial than using T3.
–Bolus administration of T4 can cause hyperkalemia, and it is advisable to administer 10 units of regular insulin and 50 mL of 50% dextrose prior to bolus administration of T4, unless the serum glucose is greater than 300 mg/dL.
–If intravenous preparation is not available, thyroxine needs to be replaced enterally.
•Methylprednisolone
–Methylprednisolone is given as 15 mg/kg IV bolus and repeated on a daily basis.
–This has been shown to improve the potential for lung donation.
•Insulin
–The aim should be to maintain the blood glucose level below 140 mg/dL. Insulin is initiated at the rate of 1 unit/h IV infusion, and the infusion rate is adjusted appropriately to achieve this target.
–Frequent blood glucose analysis (hourly) may be required to maintain the target.
