- •T. J. Djankova, a. A. Burinskaja, s. A. Zakharenkov technology of finishing textile materials
- •1. Principal views of textile fibers
- •2. Preparation of cellulose materials for dyeing and printing
- •2.1. Bleaching of cotton textiles
- •2.2. Mercerization
- •3. Application of optical bleaches
- •3.1. Optical bleaching substances
- •3.2. Test on presence of an optical bleach
- •4. Dyeing
- •4.1. Technical classification of dyes
- •4.3. Mordant dyes
- •4.4. Acid metalline dyes
- •The abovementioned recipe and procedure of dyeing are standart and can be changed and specified according to type of the equipment and also kind of coloring material.
- •4.5. Direct dyes Direct dyes may be used for dyeing cotton and other cellulose fibers. Direct dyes simple in application, are suitable for dyeing on any equipment, well combined with each other.
- •4.6. Reactive dyes
- •4.6.1. Cellulose dyeing. Batch methods of dyeing
- •Table 4.1. Dyes Bath Composition and Dyeing Conditions
- •4.6.2. Continuous dyeing
- •4.7. Cationic dyes
- •Dyeing by fast-fixing dyes
- •Dyeing of newly-formed braid
- •4.8. Disperse dyes
- •4.9. Vat dyes
- •Indigo-molecular structure Vat Yellow-molecular structure
- •Dye. . . . . . . . . . . . . . . . . . . . . .3 % from weight of a fiber
- •4.10. Sulfur dyes
- •4.11. Azo dyes synthesized in the fiber
- •5. Printing
- •5.1. Reactive dyes printing
- •5.2. Pigments printing
- •5.3. Thermoprinting of fibrous materials
- •6. Final finishing
- •6.1. Giving to fabrics of properties of water pushing away
- •6. 2. Giving to textile cloths of oil- hidrofobization
- •6.3. Giving to fabrics of fireproof properties
- •6.4. Giving to fabrics of anti-shrinkage chemical properties, form-stable finishing
- •Application Rules
- •7. Dyeing from Emulsions
- •7.1 Auxiliaries solvents
- •7.2 Emulsifiers
- •7.3 Dyeing with water-soluble dyestuffs.
- •7.4. Basic dyeable synthetic fibers
- •7.5. Physic-chemical fundamentals of emulsion technique
- •Influence of the temperature on the stability of an emulsion
- •Influence of additives on the stability of an emulsion
- •The optical properties of a water/perchloroethylene emulsion
- •Vapour pressure of a water/perchloroethylene emulsion
- •7.6 Equipment for dyeing from organic solvents
- •8. Equipment for dyeing and finishing factories.
- •8.1. Machine for washing, bleaching and dyeing “colorado”
- •8.2. Мachine «petra» f. Biancalani For obtaining effects of “worked surface”
- •8.3. High temperature machine mcs comby jigger
- •8.4. Hydraulic drying cylinder machines “jigger jht” by exclusivas tepp s.A. (Spain)
- •8.5. Vertical high-temperature high-pressure yarn dyeing plant
- •8.6. Flow line for combined bleaching and dyeing of fabrics лкб-140
- •Specification
- •8.7. Rapidstretch
- •8.8. Technodye rapid system Main features.
- •8.9. Superflux ne
- •Finally
- •8.7. Rapidstretch 84
T. J. Djankova, a. A. Burinskaja, s. A. Zakharenkov technology of finishing textile materials
Methodical manual to laboratory and practical training
St.-Petersburg
2010
УДК 677.21.027
Рекомендовано на заседании кафедры
химической технологии и дизайна текстиля
01.04.2010 г., протокол № 6
Рецензенты:
FOREWORD
The methodical manual to laboratory and practical training includes all traditional sections of discipline “Technology of fin-ishing textile materials”.
A large amount of information concerning both the chemistry and practical application of the chemicals for finishing of textile materials is shown: modern. reliable production ranges for the treatment of fabrics with optical brighteners or antistatic agents, for water- or dirt- and oil-repellent finishes, for dieing from emulsions, thermoprinting and for other finishing processes, but above all for crease-proof and wash-and wear finishing.
The Inclusion of chapters describing the theoretical principles involved will not only assist towards a fuller understanding of the practical methods described, but also will enable students and users of these materials and technologies to expand knowl-edge and to get practical skills in the field of preparation, dye-ing, printing and final furnish of fibrous materials.
Data on technology and the equipment can be used at per-formance of the tasks connected with designing of finishing manufactures.
1. Principal views of textile fibers
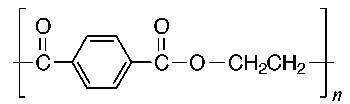
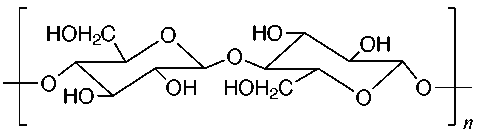
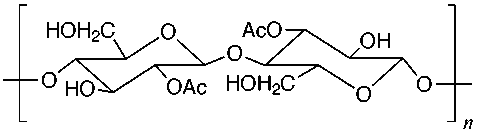
The structures of some common fibers are given below. Wool, silk, and cotton are natural fibers. Wool and silk are polypeptides, or polymers made of amino acid units. The acidic and basic amino acids present in these polymers provide many polar groups to which a dye can bind. Cotton, which is pure cellulose, has many hydroxyl groups (–OH) which can form hydrogen bonds to dyes. Rayon (or acetate) is cellulose in which some of the hydroxyl groups have been acetylated. Thus, rayon has fewer hydrogen bonding sites and is more difficult to dye than cotton. The synthetic fibers (nylon, dacron, and orlon) have fewer polar sites than the natural fibers. Nylon, a polyamide, is made by polymerizing a dicarboxylic acid and a diamine. It can be synthesized so that either –NH3+ or –COO- groups predominate at the ends of the chains. Dacron is a polyester made by polymerizing ethylene glycol and terephthalic acid. Orlon is a polymer of acrylonitrile.
Table 1.1. The structures of some common fibers
-
Fibers
Structure
Cotton, flax, hydrated cellulose fiber
Rayon (or Cellulose
Acetate)
Wool, silk
(where R = amino acid residue)
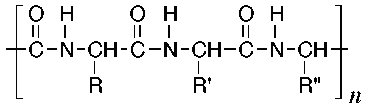
Orlon
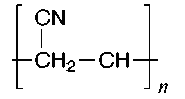
Nylon
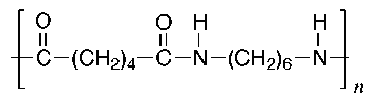
Dacron