
- •Course of lectures «Contemporary Physics: Part2»
- •Some Properties of Nuclei
- •Some Properties of Nuclei
- •Some Properties of Nuclei
- •Some Properties of Nuclei
- •Some Properties of Nuclei
- •Some Properties of Nuclei
- •Some Properties of Nuclei
- •Nuclear Binding Energy
- •Nuclear Binding Energy
- •Nuclear Models
- •Nuclear Models
- •Nuclear Models
- •Nuclear Models
- •Radioactivity
- •Radioactivity
- •Radioactivity
- •The Decay Processes
- •The Decay Processes
- •The Decay Processes
- •The Decay Processes
- •The Decay Processes
- •The Decay Processes
- •The Decay Processes
- •The Decay Processes
- •The Decay Processes
- •The Decay Processes
- •Natural Radioactivity
- •Nuclear Reactions
- •Nuclear Reactions
- •Nuclear Magnetic Resonance and
- •Nuclear Magnetic Resonance and

Course of lectures «Contemporary Physics: Part2»
Lecture №13
 Nuclear Structure. Some Properties of Nuclei. Nuclear Binding Energy. Nuclear Models.
Nuclear Structure. Some Properties of Nuclei. Nuclear Binding Energy. Nuclear Models.
Radioactivity. The Decay Processes. Natural
Radioactivity. Nuclear Reactions. Nuclear
Magnetic Resonance and Magnetic Resonance
Imaging.

 Some Properties of Nuclei
Some Properties of Nuclei
We describe the atomic nucleus by the number of protons and neutrons it contains, using the following quantities: 
A nuclide is a specific combination of atomic number and mass number that represents a nucleus.
The nuclei of all atoms of a particular element contain the same number of protons but often contain different numbers of neutrons. Nuclei related in this way are called isotopes. The isotopes of an element have the same Z value but different N and A values.

 Some Properties of Nuclei
Some Properties of Nuclei
Charge and Mass
The atomic mass unit u is defined in such a way that the mass of one atom of the isotope 12C is exactly 12 u, where 1 u is equal to 1.660 539x10-27 kg. 
 Some Properties of Nuclei
Some Properties of Nuclei
Charge and Mass
It is often convenient to express the atomic mass unit in terms of its rest-energy equivalent. For one atomic mass unit,
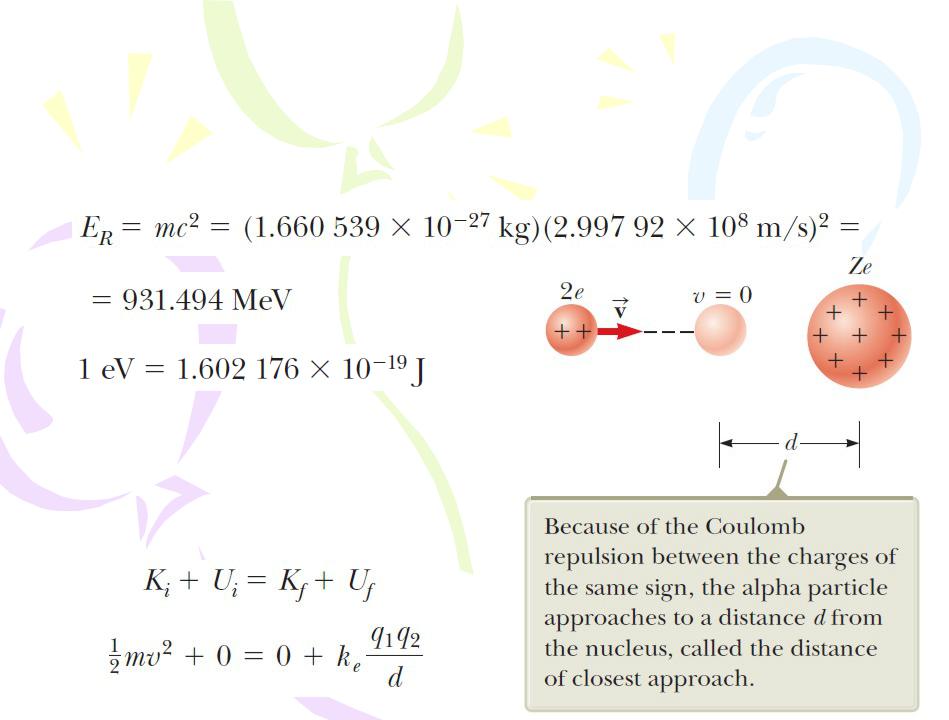
The Size and Structure of Nuclei
Applying the conservation of energy principle to the system gives
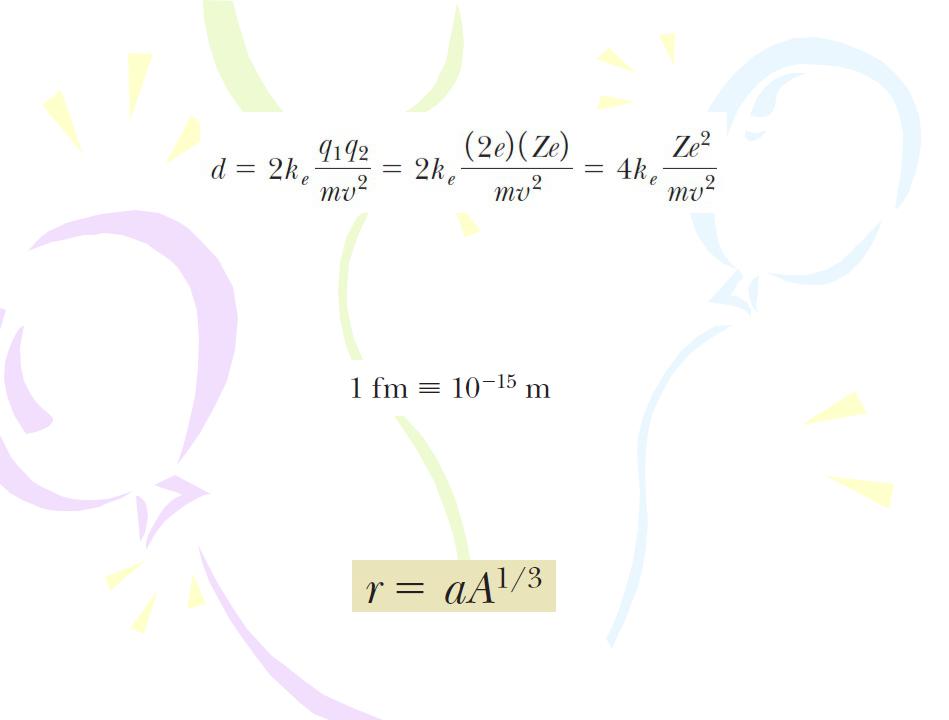
 Some Properties of Nuclei
Some Properties of Nuclei
The Size and Structure of Nuclei
Because such small lengths are common in nuclear physics, an often-used convenient length unit is the femtometer (fm), which is sometimes called the fermi and is defined as
Since the time of Rutherford’s scattering experiments, a multitude of other experiments have shown that most nuclei are approximately spherical and have an average radius given by 
 Some Properties of Nuclei
Some Properties of Nuclei
The Size and Structure of Nuclei
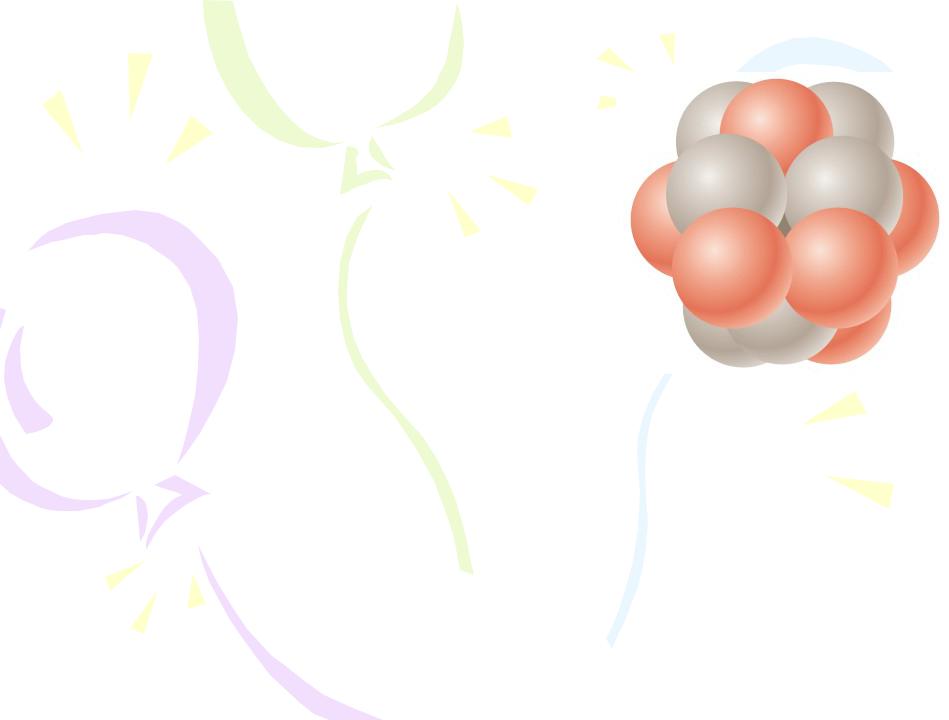
Because the volume of a sphere is proportional to the cube of its radius, the volume of a nucleus (assumed to be spherical) is directly proportional to A, the total number of nucleons. This proportionality suggests that all nuclei have nearly the same density. When nucleons combine to form a nucleus, they combine as though they were tightly packed spheres.
A nucleus can be modeled as a cluster of tightly packed spheres, where each sphere is a nucleon. 
 Some Properties of Nuclei
Some Properties of Nuclei
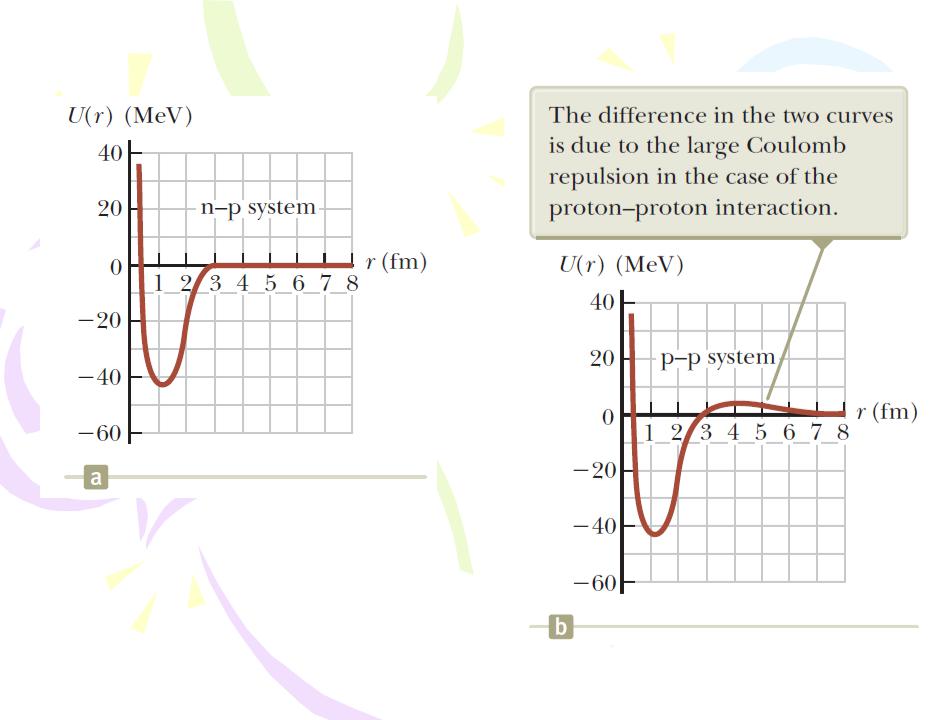
Nuclear Stability
(a) Potential energy versus separation distance for a neutron–proton system. (b) Potential energy versus separation distance for a proton–proton system. To display the difference in the curves on this scale, the height of the peak for the proton–proton curve has been exaggerated by a factor of

 Some Properties of Nuclei
Some Properties of Nuclei
Nuclear Stability
Neutron number N versus atomic number Z for stable nuclei (black dots).
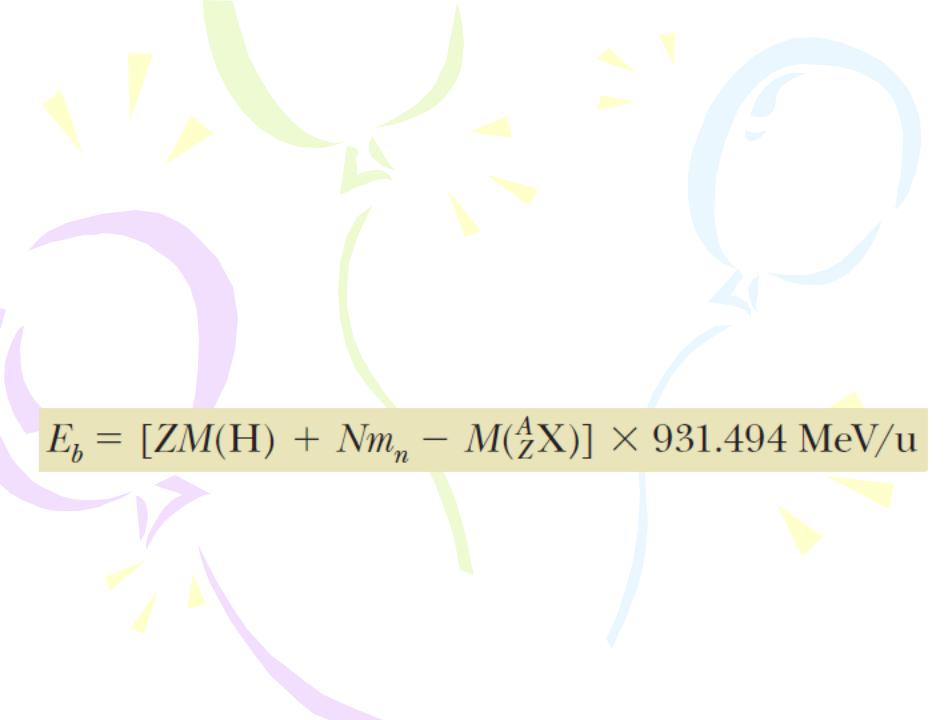
 Nuclear Binding Energy
Nuclear Binding Energy
the total mass of a nucleus is less than the sum of the masses of its individual nucleons. Therefore, the rest energy of the bound system (the nucleus) is less than the combined rest energy of the separated nucleons. This difference in energy is called the binding energy of the nucleus and can be interpreted as the energy that must be added to a nucleus to break it apart into its components. Therefore, to separate a nucleus into protons and neutrons, energy must be delivered to the system.
where M(H) is the atomic mass of the neutral hydrogen atom, mn is the mass of the neutron, M(AZX) represents the atomic
mass of an atom of the isotope AZX, and the masses are all in atomic mass units.
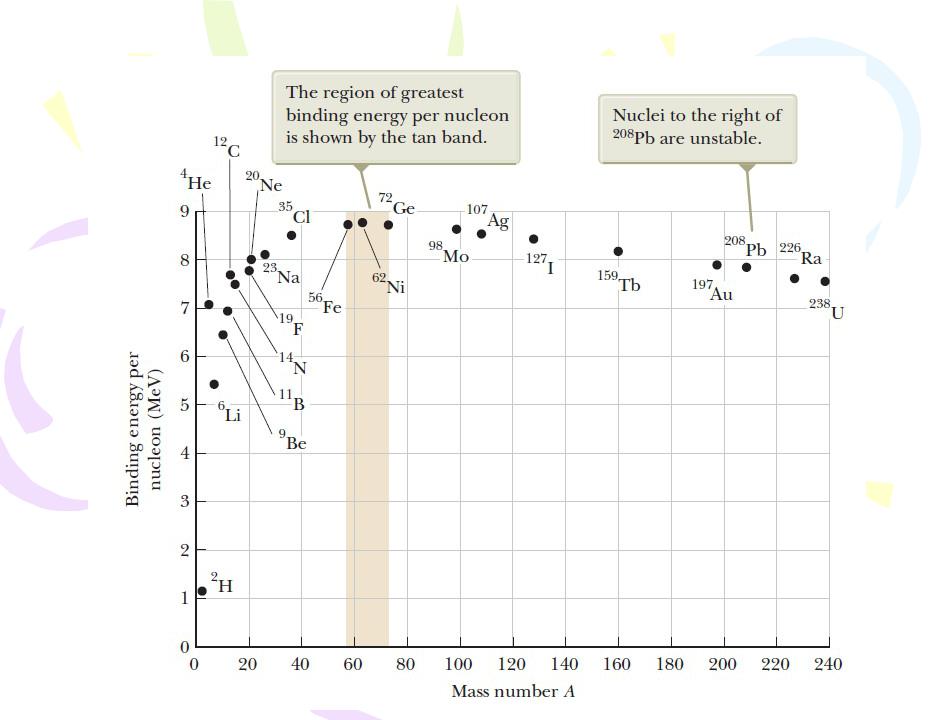

 Nuclear Binding Energy
Nuclear Binding Energy