
- •Course of lectures «Contemporary Physics: Part2»
- •The Wave Function
- •The Wave Function
- •The Wave Function
- •The Wave Function
- •The Wave Function
- •The Wave Function
- •The Wave Function
- •The Wave Function
- •SOLUTION
- •The Wave Function
- •The Wave Function
- •The Wave Function
- •Analysis Model: Quantum Particle
- •Analysis Model: Quantum Particle
- •Analysis Model: Quantum Particle A Particle in a Box Under Boundary Conditions
- •Analysis Model: Quantum Particle
- •Analysis Model: Quantum Particle A Particle in a Box Under Boundary Conditions
- •Analysis Model: Quantum Particle
- •Analysis Model: Quantum Particle
- •Analysis Model: Quantum Particle
- •The Schrödinger Equation
- •The Schrödinger Equation
- •The Schrödinger Equation
- •The Schrödinger Equation
- •The Schrödinger Equation
- •A Particle in a Well of Finite Height
- •A Particle in a Well of Finite Height
- •A Particle in a Well of Finite Height
- •A Particle in a Well of Finite Height
- •Tunneling Through a Potential Energy Barrier
- •Tunneling Through a Potential Energy Barrier
- •The Simple Harmonic Oscillator
- •The Simple Harmonic Oscillator
- •The Simple Harmonic Oscillator

Course of lectures «Contemporary Physics: Part2»
Lecture №9
Quantum Mechanics. The Wave Function.
Analysis Model: Quantum Particle Under
Boundary Conditions. The Schrödinger
Equation. A Particle in a Well of Finite Height. Tunneling Through a Potential Energy Barrier.
Applications of Tunneling. The Simple
Harmonic Oscillator.
The Wave Function
We concluded on the basis of experimental evidence that both matter and electromagnetic radiation are sometimes best modeled as particles and sometimes as waves, depending on the phenomenon being observed. We can improve our understanding of quantum physics by making another connection between particles and waves using the notion of probability
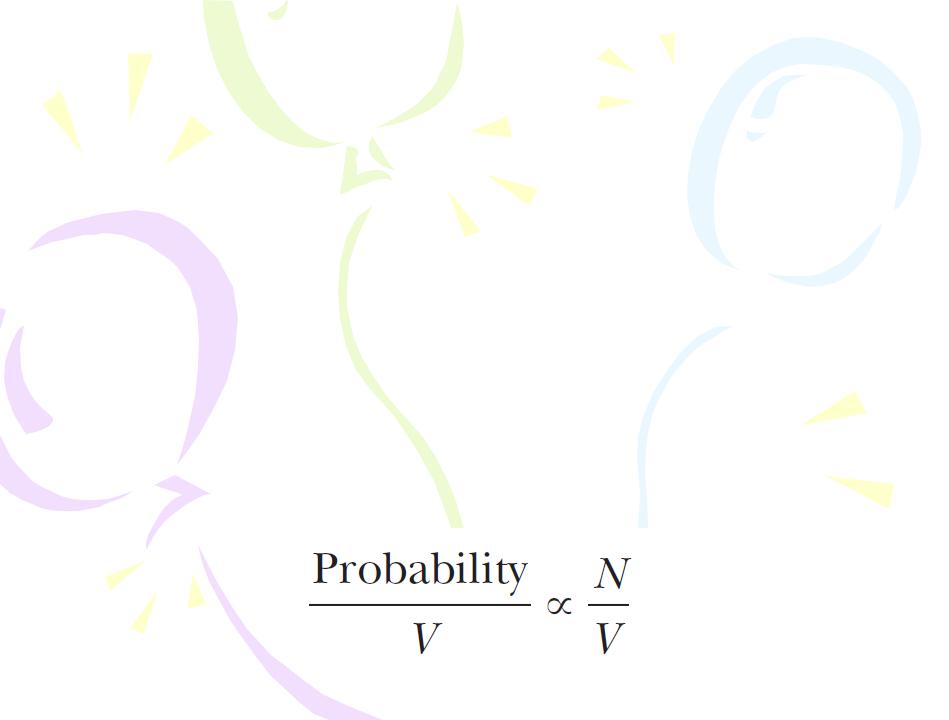
We begin by discussing electromagnetic radiation using the particle model. The probability per unit volume of finding a photon in a given region of space at an instant of time is proportional to the number of photons per unit volume at that time:


The Wave Function
The number of photons per unit volume is proportional to the intensity of the radiation: 

Now, let’s form a connection between the particle model and the wave model by recalling that the intensity of electromagnetic radiation is proportional to the square of the electric field amplitude E for the electromagnetic wave:
Equating the beginning and the end of this series of proportionalities gives
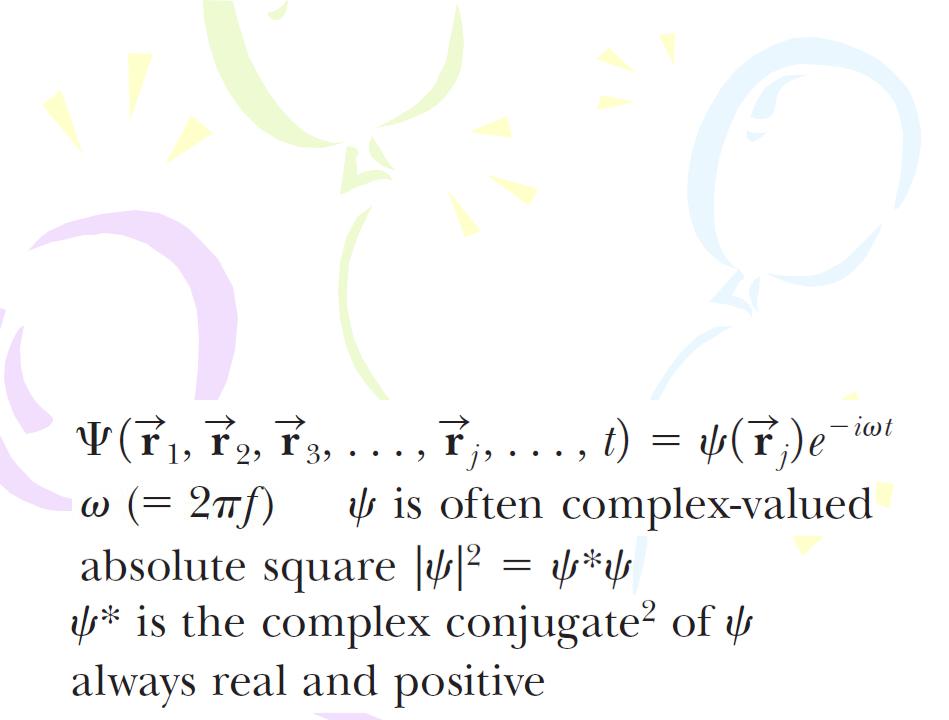
The Wave Function
The amplitude of the de Broglie wave associated with a particle is not a measurable quantity because the wave function representing a particle is generally a complex function as we discuss below. In contrast, the electric field for an electromagnetic wave is a real function. The matter analog to Equation 41.1 relates the square of the amplitude of the wave to the probability per unit volume of finding the particle. Hence, the amplitude of the wave associated with the particle is called the probability amplitude, or the wave function, and it has the symbol Ψ.
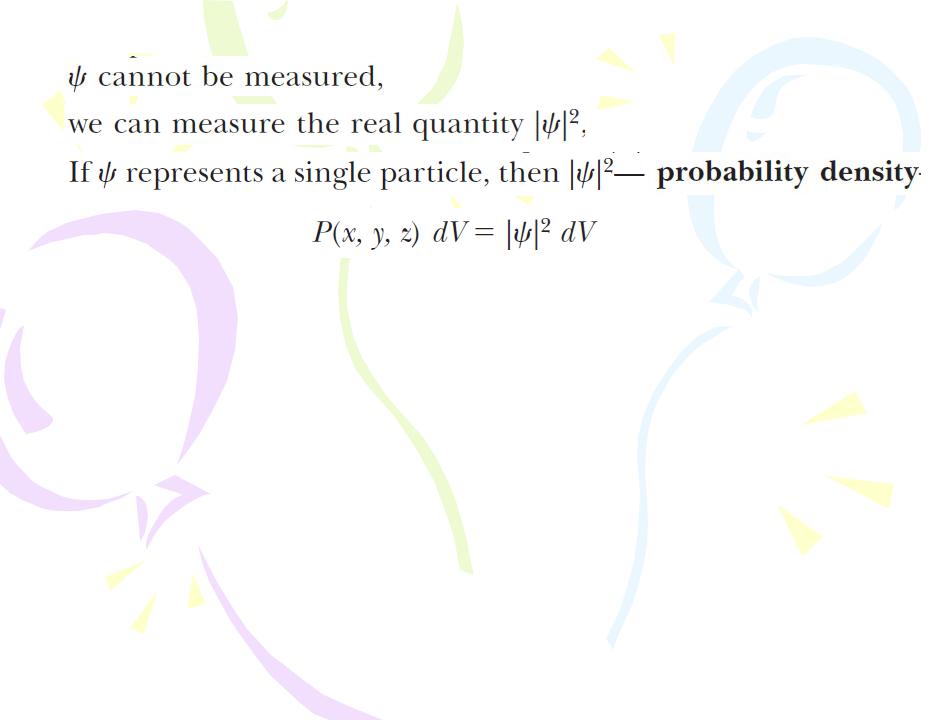
The Wave Function
This probabilistic interpretation of the wave function was first suggested by Max Born (1882–1970) in 1928. In 1926, Erwin Schrцdinger proposed a wave equation that describes the manner in which the wave function changes in space and time.
The Schrцdinger represents a key element in the theory of quantum mechanics.

The Wave Function
One-Dimensional Wave Functions and Expectation Values
The probability that the particle will be found in the infinitesimal interval dx around the point x is

The Wave Function
Although it is not possible to specify the position of a particle with complete certainty, it is possible to specify the probability of observing it in a region surrounding a given point x. The probability of finding the particle in the arbitrary interval a ≤ x ≤ b is 
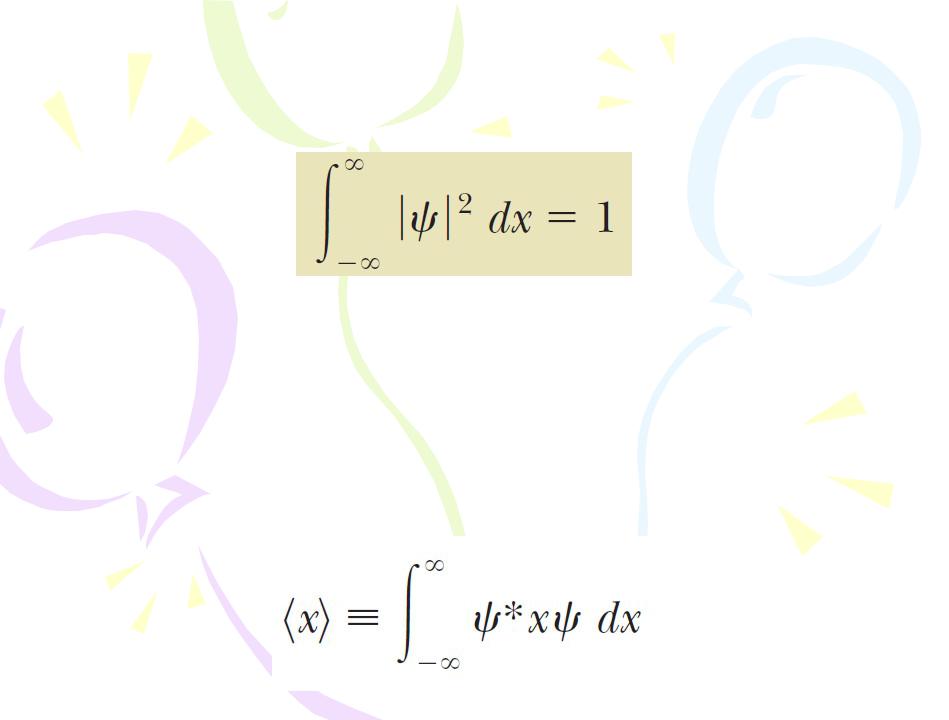
The Wave Function
Because the particle must be somewhere along the x axis, the sum of the probabilities over all values of x must be 1: 
Any wave function satisfying this Equation is  said to be normalized. Normalization is simply a statement that the particle exists at some point in space. Once the wave function for a particle is known, it is possible to calculate the average position at which you would expect to find the particle after many measurements. This average position is called the expectation value of x and is defined by the equation
said to be normalized. Normalization is simply a statement that the particle exists at some point in space. Once the wave function for a particle is known, it is possible to calculate the average position at which you would expect to find the particle after many measurements. This average position is called the expectation value of x and is defined by the equation
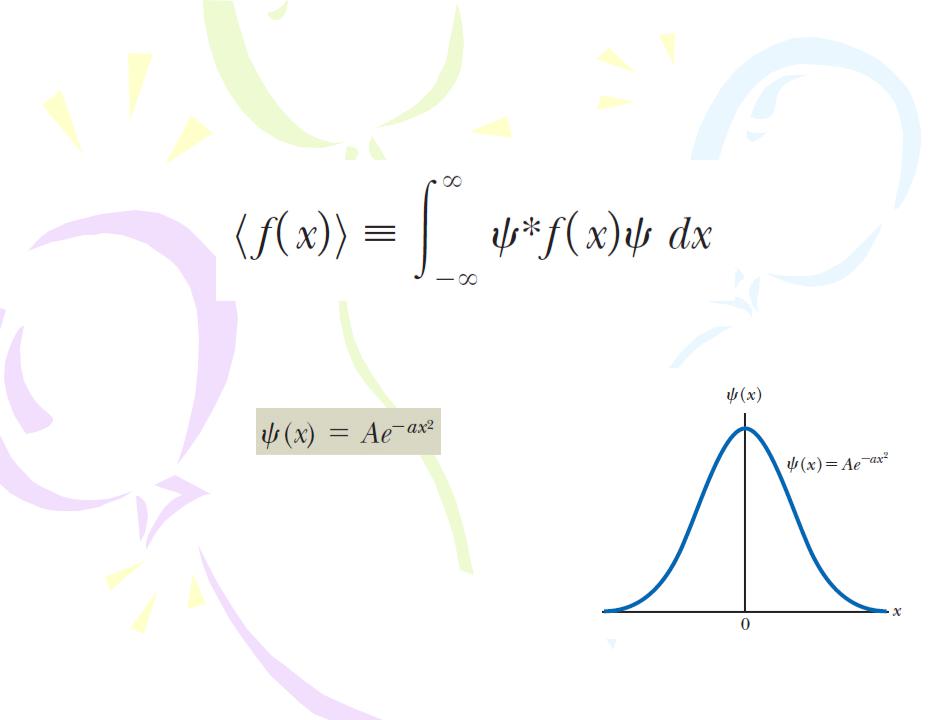
The Wave Function
Furthermore, one can find the expectation value of any function f(x) associated with the particle by using the following equation:
Example Consider a particle whose wave function is graphed in Figure and is given by
(A) What is the value of A if this wave function is normalized?
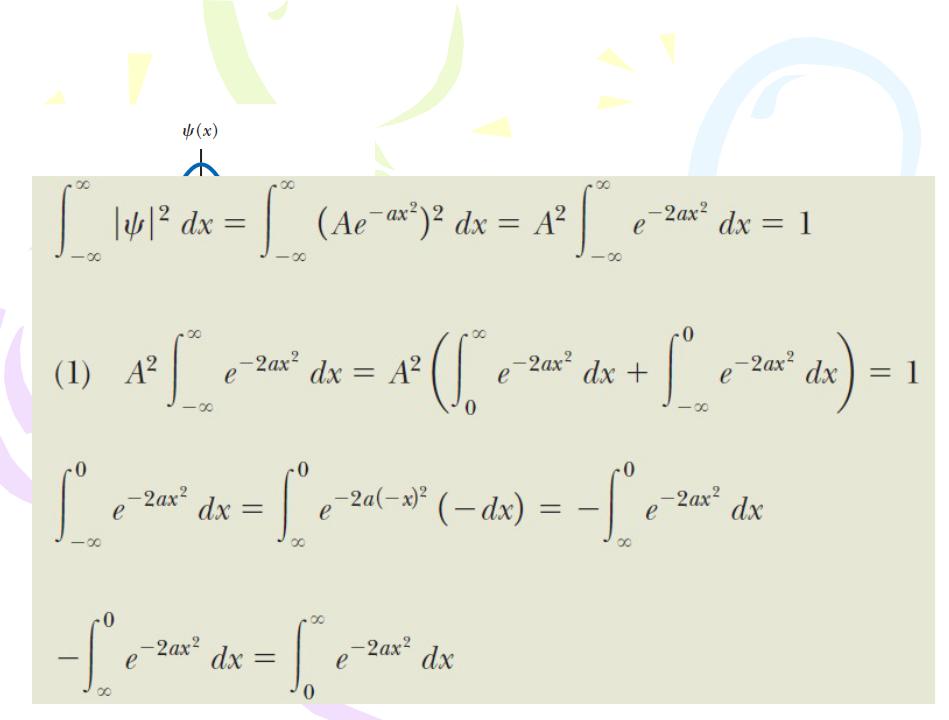
SOLUTION
The Wave Function
Apply the normalization condition, to the wave function: 
