
- •Contents
- •Contributors
- •Foreword
- •Preface
- •1. Epidemiology of recurrent pregnancy loss
- •2. Signaling between embryo and mother in early pregnancy: Basis for development of tolerance
- •3. Genetics of spontaneous abortions
- •3e. Should CVS or amniocentesis be performed in RPL without screening?
- •4. Does the maternal immune system regulate the embryo’s response to teratogens?
- •6. Endocrinology of pregnancy loss
- •7. Antiphospholipid syndrome – pathophysiology
- •8. Diagnosis of aPL-associated abortions
- •10. Defects in coagulation factors leading to recurrent pregnancy loss
- •11. Uterine anomalies and recurrent pregnancy loss
- •12. Immunobiology of recurrent miscarriage
- •13. Infections and recurrent pregnancy loss
- •16. Obstetric outcomes after recurrent pregnancy loss
- •17. Coping with recurrent pregnancy loss: Psychological mechanisms
- •19. Investigation protocol for recurrent pregnancy loss
- •20. A patient’s perspective
- •Index

16. Obstetric outcomes after recurrent pregnancy loss
Howard JA Carp
INTRODUCTION
Most work on recurrent pregnancy loss (RPL) has concentrated on the causes, prognosis, treatment, and subsequent live birth rate. However, a question arises as to whether this group of patients is at a higher risk for obstetric complications such as bleeding, fetal anomalies, preeclampsia, intrauterine growth restriction (IUGR), preterm labor, and perinatal mortality, and whether prenatal care has to be modified to seek these complications. The question then arises as to whether these complications are associated with specific conditions associated with RPL. Antiphospholipid syndrome (APS) and hereditary thrombophilias are two such conditions that have been described to be associated with recurrent pregnancy loss and late obstetric complications. Various interventions have also been reported to affect the incidence of later obstetric complications. These interventions include paternal leukocyte immunization and intravenous immunoglobulin (IVIG) for unexplained RPL, anticoagulants and aspirin or IVIG for APS, and anticoagulants for hereditary thrombophilias. This chapter assesses some of the obstetric complications associated with different forms of RPL and the treatment modalities that have been used.
METHOD OF STUDY
Publications describing the obstetric complications in RPL were sought by a thorough literature search including online databases, MEDLINE and EMBASE. The original database of the Recurrent Miscarriage Trialists Group is held by the author as one of the data contributors to that database. Figures were also
obtained from one of the author’s own databases, which contains information on 1800 patients attending the Recurrent Miscarriage Clinic of the Sheba Medical Center, Tel Hashomer, Israel. The figures were entered into a computerized database (SPSS, Chicago, Ill) and analyzed.
STATISTICAL ANALYSIS
Odds ratios (OR) with 95% confidence intervals (CI) were calculated for developing obstetric complications such as vaginal bleeding, anomalies, preeclampsia, IUGR, and perinatal mortality. When true incidences were available from cohort studies, the relative risks (RR) were calculated. These figures were also compared in subgroups of patients and after various treatment interventions.
INCIDENCE OF OBSTETRIC
COMPLICATIONS AFTER RPL
Most of the literature on obstetric complications comes from an era after APS had been defined but before hereditary thrombophilias had been defined. Reginald et al,1 in a retrospective observational cohort study, assessed the results of 175 pregnancies in 97 recurrently miscarrying women whose subsequent pregnancy progressed beyond 28 weeks. However, the underlying causes of RPL in this group of women were not documented. The results were not compared with a control group attending the same hospital, but with standard figures from Scotland between 1973 and 1979. A significantly higher incidence of preterm deliveries, perinatal deaths, and IUGR was found. In contrast, Hughes et al2 examined the obstetric
231

RECURRENT PREGNANCY LOSS: CAUSES, CONTROVERSIES AND TREATMENT
outcome in 88 women with a past history of three or more consecutive pregnancy losses and compared the results with a control group drawn from their local obstetric population. The incidences of small-for-gestational-age infants (3.4%), preterm delivery (12.5%), and perinatal mortality (0%) were no different to those in the control group. As in the study by Reginald et al,1 there was no mention of APS. However, an increased incidence of gestational diabetes and pregnancy-induced hypertension was found. Tulppala et al3 conducted a prospective study of 32 deliveries in 63 women with RPL and presented the results of a detailed investigative protocol, including APS. The incidences of IUGR (20%), preterm delivery (9.7%), and impaired glucose tolerance (22.8%) appeared to be increased. Unfortunately, the results were not compared with any control population. Jivraj et al4 studied a cohort of 162 women with RPL compared with local controls, and found an increased incidence of the same complications as Reginald et al,1 but also an increased incidence of cesarean sections, which were performed for the above obstetric conditions. Although that study did define the causes of pregnancy loss in the control group, the figures were too small to allow comparisons to be drawn between different groups of patients. Seidman et al5 reported the same complications in 338 women with pregnancy losses compared with 13 338 parous women in a multicenter study in four large hospitals in Jerusalem. In the survey by Seidman et al,5 there was a statistically significant 14% prevalence of preterm labor, compared with 7.5% in control women (OR 2.32; 95% CI 1.67–3.22). The different prevalence of IUGR was even more significant: 14% in aborting women, compared with 5% in control women (OR 3.02; 95% CI 2.17–4.18). However, the study by Seidman et al5 included patients with one pregnancy loss and was not restricted to patients with RPL. A more recent population-based study has been reported by Sheiner et al,6 in which all singleton pregnancies were assessed in women with and without two or more consecutive recurrent abortions. Between 1988 and 2002, 154 294 singleton deliveries occurred. Of these deliveries, 7503 occurred in
patients with RPL. The following complications were found to be associated with RPL: advanced maternal age, cervical incompetence, diabetes mellitus, hypertensive disorders, placenta praevia and abruptio placentae, malpresentations, and premature rupture of the membranes (PROM). A higher rate of cesarean section was also found in patients with previous RPL compared with controls: 15.9% and 10.9%, respectively (OR 1.6; 95% CI 1.5–1.7).
An attempt was made to determine the common OR for various late complications of pregnancy after RPL compared with controls. The series of Reginald et al1 could not be included as there was no relevant control group. The series of Seidman et al5 was not included as it only described patients with one previous miscarriage. The series of Tulppala et al3 could not be included as there was no control group. The other three publications were combined in a meta-analysis.
VAGINAL BLEEDING
The incidence of bleeding was only described in the series of Reginald et al quoted by Beard,7 and in that of Seidman et al.5 Hence no common OR could be calculated. Vaginal bleeding seems to be increased in pregnancies that develop. Vaginal bleeding is a common complication, occurring in 50 of 162 women in the series of Reginald et al7 and in 50 of 102 patients in the author’s series.8 The reason for this bleeding remains unclear. Seventyfive percent of habitual abortions are blighted ova.8 However, when the pregnancy succeeds and there is a live embryo within the uterus, bleeding still occurs in 40–50% of patients. Seidman et al5 also reported a 13.7% incidence of first-trimester bleeding, compared with a 13.8% incidence in the standard population.
ANOMALIES
Little information is available on anomalies. The study by Sheiner et al6 reports 2 anomalies in 29 patients. Although this is a very small series, the figures are higher than expected. Analysis of the figures in the RMITG trial9 showed an anomaly rate
232
OBSTETRIC OUTCOMES AFTER RECURRENT PREGNANCY LOSS
Study |
Statistics for each study |
OR and 95% Cl |
|
|
||||||||||||
|
|
Lower |
Upper |
|
|
|
|
|
|
|
|
|
|
|
|
|
Hughes et al2 |
OR |
limit |
limit |
p |
|
|
|
|
|
|
|
|
|
|
|
|
7.001 |
3.977 |
12.323 |
0.000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Jivraj et al4 |
2.335 |
0.739 |
7.379 |
0.149 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Sheiner et al6 |
2.233 |
2.073 |
2.406 |
0.000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Common OR |
2.277 |
2.116 |
2.451 |
0.000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
0.1 |
1 |
10 |
100 |
|||||||||
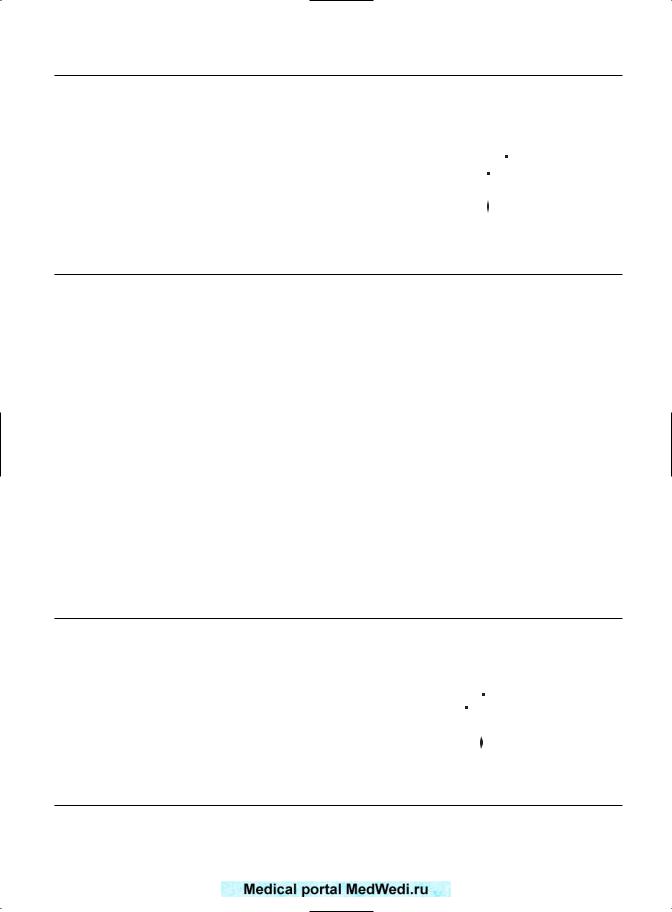
Figure 16.1 Odds ratio (OR) for gestational diabetes in recurrent pregnancy loss.
of 4%. In the author’s series, there were three anomalies in 99 developing pregnancies in non-treated patients. However, in the RMITG series and the author’s series, no control group is available.
DIABETES
Three papers have described the prevalence of diabetes: Hughes et al,2 Jivraj et al,4 and Sheiner et al.6 As a control group was available, their common OR could be determined for 7753 patients with RPL and 172 490 control patients. The prevalence of diabetes was 11.75% and 4.95% in recurrently miscarrying and control patients, respectively. The OR are summarized in Figure 16.1. As can be seen, there was a common OR of 2.27 for gestational diabetes in RPL. This figure was statistically
significant (95% CI 2.1–2.45). Tulppala et al3 also found a prevalence of 22.6% (7 of 31 patients tested).
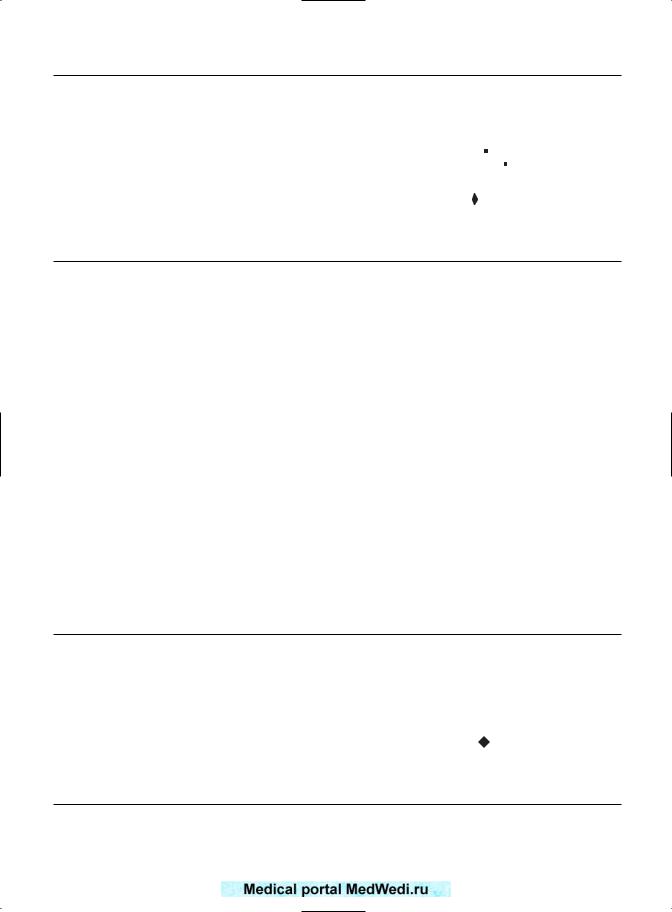
PREGNANCY-INDUCED HYPERTENSION
Hughes et al,2 Jivraj et al,4 and Sheiner et al6 quoted the incidence of pregnancy-induced hypertension (PIH). The figures are summarized in Figure 16.2. In order to obtain significant numbers, the figures for preeclampsia and other forms of PIH were analyzed as a whole. The common OR was 1.89 (95% CI 1.74–2.06), which was statistically significant. The total number of patients was identical to those with gestational diabetes. The prevalence of PIH was 671 of 7753 patients (8.6%) of recurrently miscarrying patients, compared with 5.7% of control women (9921 of 172 490 control patients).
Study |
Statistics for each study |
OR and 95% Cl |
||||||||||||||
|
|
Lower |
Upper |
|
|
|
|
|
|
|
|
|
|
|
|
|
Hughes et al2 |
OR |
limit |
limit |
p |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
1.941 |
0.783 |
4.814 |
0.152 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Jivraj et al4 |
0.728 |
0.412 |
1.285 |
0.274 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Sheiner et al6 |
1.933 |
1.778 |
2.102 |
0.000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Common OR |
1.894 |
1.743 |
2.057 |
0.000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.1 |
1 |
10 |
100 |
|||||||||
Figure 16.2 Odds ratio (OR) for pregnancy-induced hypertension in recurrent pregnancy loss.
233
RECURRENT PREGNANCY LOSS: CAUSES, CONTROVERSIES AND TREATMENT
Study |
Statistics for each study |
OR and 95% Cl |
|||||||||||||
|
|
Lower |
Upper |
|
|
|
|
|
|
|
|
|
|
|
|
Hughes et al2 |
OR |
limit |
limit |
p |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
2.433 |
0.762 |
7.768 |
0.133 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Jivraj et al4 |
6.885 |
4.318 |
10.978 |
0.000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Sheiner et al6 |
1.000 |
0.847 |
1.181 |
0.998 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Common OR |
1.257 |
1.076 |
1.467 |
0.004 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.1 |
1 |
10 |
100 |
||||||||
Figure 16.3 Odds ratio (OR) for intrauterine growth restriction in recurrent pregnancy loss.
Neither Reginald et al1 nor Tulppala et al3 quoted figures for PIH.
INTRAUTERINE GROWTH RESTRICTION
The same three papers as above give figures for IUGR. The common OR for developing IUGR is 1.25 (95% CI 1.08–1.47) (Figure 16.3). Reginald et al1 reported a 33% incidence in 344 pregnancies prospectively followed up, and reported that this showed a relative risk of 3 compared with the standard Scottish population. Tulppala et al3 also reported a 20% incidence (6 out of 30 pregnancies).
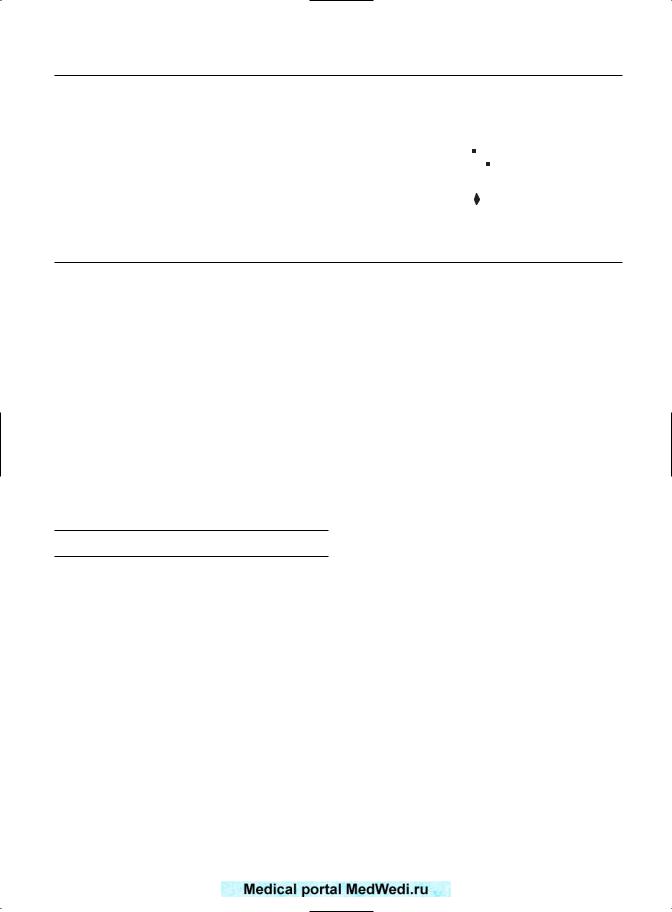
PRETERM LABOR
Comparative figures are only available for preterm labor from the series of Hughes et al2 and Jivraj et al.4
Figure 16.4 summarizes the results. Again, there was a statistically significant association between preterm labor and RPL. The common OR was 2.84 (95% CI 1.96–4.1). In the series of Reginald et al,1 there was a 28% incidence of preterm labor. The relative risk was 3.3 when compared with the Scottish data for the equivalent period. Tulppala et al3 quoted a 9.7% incidence in their series.
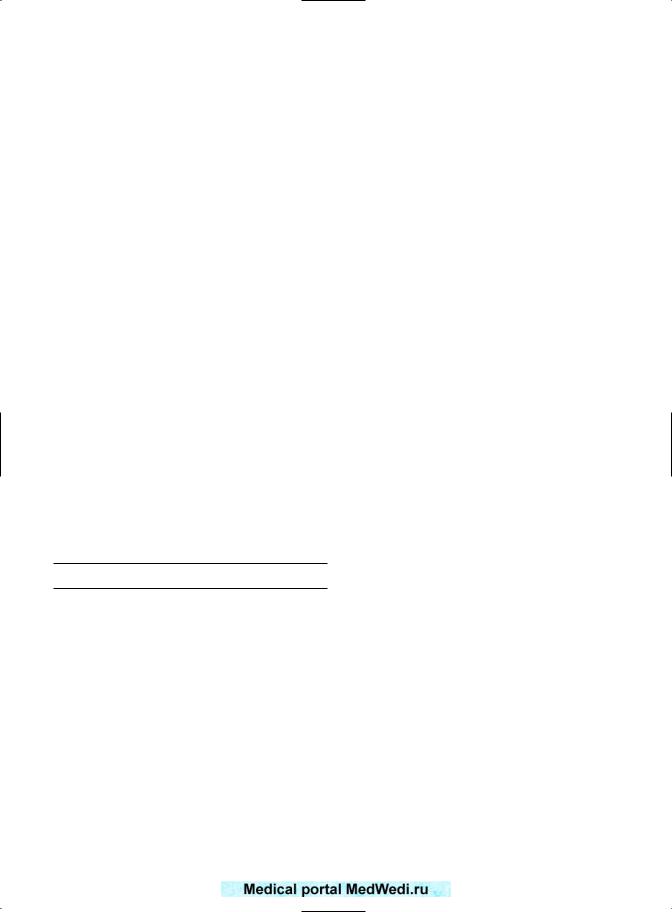
PERINATAL MORTALITY
Again, there was an increased tendency for mortality after RPL, loss with a common OR of 1.25 (95% CI 1.05–1.49). This is summarized in Figure 16.5. However, the perinatal mortality may be artificially low due to obstetric intervention for other complications in pregnancy. Reginald et al1 reported 19 perinatal deaths in 118 infants (16.1%), which
Study |
Statistics for each study |
OR and 95% Cl |
||||||||||||||||
|
|
Lower |
Upper |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OR |
limit |
limit |
p |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hughes et al2 |
1.530 |
0.811 |
2.887 |
0.189 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Jivraj et al4 |
3.890 |
2.470 |
6.126 |
0.000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Common OR |
2.837 |
1.961 |
4.104 |
0.000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.1 |
1 |
|
10 |
100 |
||||||||||
Figure 16.4 Odds ratio (OR) for preterm labor in recurrent pregnancy loss.
234
OBSTETRIC OUTCOMES AFTER RECURRENT PREGNANCY LOSS
Study |
Statistics for each study |
OR and 95% Cl |
||||||||||||
|
|
Lower |
Upper |
|
|
|
|
|
|
|
|
|
|
|
Hughes et al2 |
OR |
limit |
limit |
p |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
1.103 |
0.060 |
20.305 |
0.947 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Jivraj et al4 |
2.506 |
0.922 |
6.814 |
0.072 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Sheiner et al6 |
1.222 |
1.021 |
1.464 |
0.029 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
Common OR |
1.250 |
1.047 |
1.492 |
0.014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.1 |
1 |
10 |
100 |
|||||||
Figure 16.5 Odds ratio (OR) for perinatal mortality in recurrent pregnancy loss.
was considerably higher than the standard figures for England and Wales in the same period of time, when the perinatal mortality was 10.1/1000. Tulppala et al3 did not quote perinatal mortality.
It seems, therefore, that the currently available literature on the obstetric and neonatal outcome of pregnancies from women with a history of RPL shows a consistently worse prognosis. However, it is unclear whether the worse prognosis is only found in patients with predisposing causes such as APS and hereditary thrombophilias or is also present in patients with unexplained pregnancy losses.
ANTIPHOSPHOLIPID SYNDROME
Most series on APS have concentrated on the subsequent live birth rate and ignored the late obstetric complications. The present author published a series10 in which the outcome of 24 pregnancies in patients with lupus anticoagulant (LA) and five or more first-trimester abortions was compared with 22 pregnancies in women with no antiphospholipid antibodies (aPL), and 5 or more miscarriages. Although the subsequent number of first-trimester miscarriages and the live birth rate were similar in both groups of patients, the incidences of secondor third-trimester fetal deaths, IUGR, and need for premature induction of labor or preterm cesarean section were significantly higher in the APS patients. The similar live birth
rate was only obtained by early obstetric intervention to prevent intrauterine fetal deaths. A number of other publications have attested that the risk of obstetric complications is high in APS.11,12 The incidence of preeclampsia is particularly high in APS.13,14 IUGR has been reported with a frequency ranging from 30% to 12% in different series. Some series show a significant increase in the incidence of IUGR,15,16 whereas others have not confirmed the increased incidence.17,18 The different results on the association of obstetric complications with aPL might have various explanations. None of the results has been correlated with β2-glycoprotein I(β2GPI)-dependent antibodies. None has corrected for fetal chromosomal aberrations, in which the aPL may just be an epiphenomenon. Alternatively, the obstetric complications may be dependent on other factors associated with RPL rather than aPL. No series has compared the incidence of obstetric complications in RPL due to APS and in RPL without APS. The incidence of preterm labor is increased in APS patients,16 but again has not been compared with patients with RPL and no APS.
The currently accepted optimal treatment regimen for APS is heparin or low-molecular-weight heparin (LMWH) with the addition of low-dose aspirin. However, anticoagulants do not seem to lower the incidence of obstetric complications associated with this syndrome.19 IVIG may have a beneficial effect in APS, as the action and production of aPL are inhibited by IVIG. The F(ab′) fragment of IVIG
235
RECURRENT PREGNANCY LOSS: CAUSES, CONTROVERSIES AND TREATMENT
inhibits binding of anticardiolipin antibody (aCL) to cardiolipin in a dose-dependent manner.20 The F(ab′) fragment of IVIG inhibits LA activity.21 IVIG lowers levels of aCL after each infusion.22 IVIG may contain anti-idiotypic antibodies to aPL, or may inactivate B-cell clones, leading to decreased autoantibody production.23 In the late 1980s and early 1990s, IVIG was used to improve the live birth rate in APS.24-27 However, these were small series, and showed IVIG to have no apparent benefit over anticoagulants in terms of live births. However, when the obstetric complications are considered, a different clinical picture emerges. Vaquero et al28 compared IVIG with prednisone and aspirin. The prevalence of IUGR and preterm labor was similar in both groups, but the prevalences of PIH and gestational diabetes were significantly lower (p<0.05) after IVIG (5% of 41 patients and 14% of 22 patients, respectively). Branch et al29 compared IVIG with placebo: there were fewer cases of IUGR after IVIG (14% of 7 patients, compared with 33% of 9 patients, respectively), and fewer admissions to the neonatal intensive care unit (14% of 7 patients after IVIG, compared with 44% of 9, respectively). Harris and Pierangelli30 reported that preeclampsia, IUGR and prematurity were reduced when IVIG was compared with prednisone and aspirin or heparin and aspirin.
HEREDITARY THROMBOPHILIAS
There have recently been numerous publications associating genetic predispositions (hereditary thrombophilias) to thrombosis with pregnancy loss. The hereditary thrombophilias include protein C, protein S, and antithrombin III deficiencies, activated protein C resistance (APCR), factor V Leiden mutation, methylenetetrahydrofolate reductase (MTHFR) mutation, the prothrombin gene mutation (G20210A) and excessive factor VIII. The features of hereditary thrombophilias and the effects on obstetric complications are discussed in Chapter 10. Suffice it to say here that Kupferminc et al31 have reported an association between
thrombophilias and severe preeclampsia, placental abruption, IUGR, and stillbirth. However, theirs was a case–control study looking at the prevalence of hereditary thrombophilias in women with obstetric complications. Additionally, these findings have beeen disputed by Infante-Rivarde et al,32 who also performed a case–control study. Alfirevic et al33 have published a systematic review of the various reports and have described the strengths of association between hereditary thrombophilias and preeclampsia, placental abruption, stillbirth, and IUGR. Sheiner et al6 have drawn attention to the fact that the other publications on obstetric complications in RPL were written at a time when the hereditary thrombophilias had not yet been recognized. In their series, higher rates of IUGR, cesarean section, low Apgar scores and perinatal mortality were found among the 22 patients with known thrombophilia as compared with the controls, although the differences did not reach statistical significance. There are no cohort studies assessing the true incidence of obstetric complications in the presence of thrombophilia and RPL. In the present author’s series of 21 pregnancies with factor V Leiden which were followed up prospectively, there was one case of HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count), but no other obstetric complications, and no deep vein thrombosis or pulmonary embolus.
The LMWH enoxaparin has been shown to be associated with a significantly increased chance of a live birth, both overall and when corrected for the number of miscarriages34 (RR 0.53; 95% CI 1.07–3.28), in women with RPL and hereditary thrombophilia. There are isolated reports35–37 of the use of anticoagulants in the presence of thrombophilias to reduce the late obstetric complications, and this approach has been endorsed by the American College of Chest Physicians.38 However, there is as yet insufficient evidence that anticoagulants actually reduce the incidence of late obstetric complications. Further trials need to be performed in order to determine whether anticoagulants do indeed reduce the incidence of obstetric complications.
236

OBSTETRIC OUTCOMES AFTER RECURRENT PREGNANCY LOSS
OBSTETRIC COMPLICATIONS AFTER |
|
Table 16.1 Preterm labor, IUGR, perinatal |
|
|||||
mortality, and anomalies with paternal leukocyte |
||||||||
ALLOIMMUNIZATION |
immunization compared with controlsa |
|
||||||
|
|
|
|
|
|
|
||
Some 20 years ago, alloimmunization had become |
|
Immunized |
Controls |
|
|
|||
|
(n = 979)b |
(n = 483)b |
Relative riskc |
|||||
the treatment of choice for patients with RPL. |
|
|||||||
|
|
|
|
|
|
|||
Two methods of alloimmunization had been used |
Preterm labor |
39 (3.9%) |
52 (10.8%) |
0.63 (0.49–0.79) |
||||
in order to improve the subsequent live birth rate in |
IUGR |
17 (1.7%) |
59 (12.2%) |
0.32 (0.21–0.49) |
||||
recurrently aborting women: active immunization |
Perinatal mortality |
9 (0.9%) |
21 (4.3%) |
0.44 (0.26–0.47) |
||||
Anomalies |
25 (2.6%) |
19 (3.9%) |
0.84 (0.65–1.10) |
|||||
with paternal leukocytes and passive immuniza- |
||||||||
|
|
|
|
|
|
|||
aFigures include 92 immunized and 175 non-immunized patients from |
||||||||
tion using IVIG.39 Paternal leukocyte immunization |
||||||||
7 |
|
|
|
|
|
|||
became standard management after the double- |
Beard’s series , 759 immunized patients and 279 non-immunized |
|||||||
patients from the RMITG register,9 and 128 immunized patients and 29 |
||||||||
blinded randomized trial by Mowbray et al40 and |
non-immunized patients from the present author’s series. |
|
||||||
a subsequent meta-analysis by the Recurrent |
bIncidences in parentheses. |
|
|
|
|
|||
c95% confidence intervals in parentheses. |
|
|
||||||
Miscarriage Immunization Trialists Group,9 but fell |
|
|
|
|
|
|
||
out of favor due to the paper by Ober et al,41 which |
|
|
|
|
|
|
||
claimed lack of efficacy. As with treatment for APS |
These figures are summarised in Table 16.2. As can |
|||||||
and hereditary thrombophilias, most publications |
||||||||
be seen, the incidence of preeclampsia was lower |
||||||||
on immunomodulation have assessed the subsequent |
||||||||
in immunized |
women. However, |
there |
was no |
|||||
live birth rate as the primary outcome measure, and |
||||||||
significant difference |
in |
the incidence of |
vaginal |
|||||
have ignored the late obstetric complications of |
||||||||
bleeding, which remained high: 38% and 34% in |
||||||||
pregnancy occurring in women with a subsequent |
||||||||
immunized and control women, respectively. |
||||||||
live birth. |
||||||||
Table 16.3 |
shows |
the |
obstetric |
complications |
||||
In order to summarize the obstetric complica- |
||||||||
after IVIG in the 136 women in the present author’s |
||||||||
tions with or without immunotherapy, the various |
||||||||
series. There is no relevant control group, as some |
||||||||
series in the literature have been pooled to obtain |
||||||||
of the patients were administered IVIG after failure |
||||||||
a sufficient number of patients available for mean- |
||||||||
of paternal leukocyte |
immunization. The |
figures |
||||||
ingful analysis. The databases of Beard et al,7 the |
||||||||
were compared with |
those for non-immunized |
|||||||
RMITG meta-analysis9 and the present author’s |
||||||||
patients in the registers above and those in control |
||||||||
series have been combined in order to compare the |
||||||||
patients in other series on IVIG in the literature. The |
||||||||
obstetric complications after paternal leukocyte |
||||||||
incidences of IUGR, perinatal mortality, bleeding, |
||||||||
immunization: 979 immunized patients were avail- |
||||||||
|
|
|
|
|
|
|||
able for analysis, compared with 483 non-immunized |
|
|
|
|
|
|
||
patients. The prevalence of IUGR, perinatal mortal- |
|
|
|
|
|
|
||
ity, and the incidence of anomalies were assessed. |
Table 16.2 Bleeding and preeclampsia with |
|
||||||
These figures are summarized in Table 16.1. As can |
|
|||||||
paternal leukocyte immunization compared |
|
|||||||
be seen, there was a significantly lower incidence of |
with controlsa |
|
|
|
|
|
||
preterm labor, perinatal mortality, and IUGR after |
|
|
|
|
|
|
||
|
Immunized |
Controls |
|
|
||||
paternal leukocyte immunization than in controls. |
|
|
|
|||||
|
(n = 216)b |
(n = 191)b |
Relative riskc |
|||||
The incidence of fetal anomalies was not sig- |
|
|
|
|
|
|
||
|
|
|
|
|
|
|||
nificantly different in the two groups of patients. |
Bleeding |
83 (38%) |
|
64 (34%) |
1.10 (0.92–1.33) |
|||
As the RMITG meta-analysis9 did not assess bleed- |
Preeclampsia |
27 (13%) |
|
57 (30%) |
0.55 (0.40–0.76) |
|||
ing and preeclampsia, data could only be obtained |
aFigures include 88 immunized and 162 non-immunized patients from |
|||||||
from Beard’s7 series and the present author’s series. |
Beard’s series7 and 128 immunized patients and 29 non-immunized |
|||||||
Two hundred and sixteen immunized patients |
patients from the present author’s series. |
|
|
|||||
bIncidences in parentheses. |
|
|
|
|
||||
and 191 control patients are available for analysis. |
c95% confidence intervals in parentheses. |
|
|
|||||
237
RECURRENT PREGNANCY LOSS: CAUSES, CONTROVERSIES AND TREATMENT
Table 16.3 Obstetric complications after
IVIG compared with controlsa
|
IVIGb |
Controlsb |
Relative riskc |
|
|
|
|
Preterm labor |
15/136 (11%) |
52/483 (10.8%) |
1.02 (0.53–1.96) |
IUGR |
6/136 (4%) |
59/483 (12.2%) |
0.39 (0.18–0.86) |
Perinatal |
2/136 (1.5%) |
21/483 (4.3%) |
0.39 (0.10–0.47) |
mortality |
|
|
|
Bleeding |
5/136 (3.7%) |
64/191 (34%) |
0.14 (0.06–0.33) |
Preeclampsia |
4/136 (2.9%) |
57/191 (30%) |
0.13 (0.05–0.34) |
Anomalies |
1/136 (0.7%) |
19/483 (3.9%) |
0.22 (0.03–1.51) |
aFigures show the incidence of anomalies as a function of the total number of patients in the sample. IVIG figures are from the present author’s series. The control figures are pooled data from the literature. bIncidences in parentheses.
c95% confidence intervals in parentheses.
and preeclampsia were all lower after IVIG, but the incidences of preterm labor and anomalies did not seem to be affected. However, these results should be interpreted with caution due to the nature of the control group.
CYTOKINES AS MEDIATORS OF PREGNANCY LOSS AND OBSTETRIC COMPLICATIONS
Cytokines are low-molecular-weight peptides or glycopeptides, which are produced by lymphocytes, monocytes/macrophages, mast cells, eosinophils, and blood vessel endothelial cells. Cytokines seem to influence all stages of pregnancy. Cytokines such as interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and epidermal growth factor (EGF) stimulate placental cell proliferation42 in vitro, and may enable the trophoblast to secrete human chorionic gonadotropin(hCG) and human placental lactogen (hPL). Cytokines such as transforming growth factor β2 (TGF-β2) can inhibit lymphokine activation of natural killer (NK) cells (which may attack the trophoblast). Although cytokines can have pleiotropic actions, these actions of the above cytokines are classified as T-helper 2 (Th2) or anti-inflammatory in nature, and they seem to benefit the development of pregnancy. Other cytokines can have actions that are known as Th1 or proinflammatory, and these may be
detrimental to pregnancy; for example, tumor necrosis factor α (TNF-α) can activate NK cells at the fetomaternal interface,43 leading to apoptosis and trophoblast cell death.44 Wegman23 suggested that appropriate cytokines are necessary in order to confer benefits on the developing fetoplacental unit. In early pregnancy, the conceptus and placental tissues produce a variety of Th1-type cytokines, including interferons, interleukins and TNF.45 In normal pregnancy, the maternal immune system seems to modulate the cytokine pattern to preferential expression of Th2 cytokines, including GM-CSF, IL-3, IL-4, IL-5, IL-10, and IL-13, that may enhance fetal survival.46 Preferential expression of Th1 cytokines (TNF-α, interferon-γ (IFN-γ), and IL-2) may result in abnormal placental and embryonic growth and subsequent fetal demise.
An inappropriate cytokine balance has been reported to act in early pregnancy, causing NK-cell activation,43 placental apoptosis,44 teratogenesis,47 and excessive coagulation, particularly TNF-α and IL-6.48 Hence, cytokine imbalance is among the mechanisms that have been proposed to underlie RPL. The effect of cytokine imbalance on coagulation may explain some of the effects of hereditary thrombophilias. Additionally, APS and most of the late complications of pregnancy have also been shown to have an underlying cytokine basis.
Although it is generally accepted that aPL may act by causing blood coagulation in decidual vessels, cytokines may also be responsible for the action of aPL early in the pathogenesis of the condition. Serum TNF-α is increased in women with APS.49 IL-3 is decreased in APS,50 and fetal loss can be prevented in experimental APS by in vivo administration of recombinant IL-3.51 Alteration of the Th1/Th2 balance may also be involved in the effect of anti-idiotypic antibodies on APS.52
Late obstetric complications have also been shown to be associated with altered cytokine levels. Preeclampsia is associated with reduced IL-10 and higher IL-2 production from peripheral blood mononuclear cells (PBMC),53,54 and high serum IL-8 and TNF-α.55 In preterm births, cytokine involvement has been reported,56 particularly increased amniotic IL-6, IL-8, and TNF-α.57–60
238

OBSTETRIC OUTCOMES AFTER RECURRENT PREGNANCY LOSS
In IUGR, TGF-β in cord blood and mRNA for IL-10 are significantly reduced, whereas IL-8 mRNA is significantly higher61,62 and placental TNF-α secretion is enhanced.62
The various interventions used for improving the live birth rate in RPL may exert their effects by modulating cytokine balance, and this modulation may also influence the incidence of later obstetric complications. As stated in Chapter 10 it is possible that heparin or enoxaparin may work by antiinflammatory mechanisms rather than anticoagulation. Heparin increases serum TNF-binding protein, hence protecting against systemic harmful manifestations.63 LMWH inhibit TNF-α production.64 Thrombosis results in an inflammatory response of the vein wall. Both heparin and LMWH limit the anti-inflammatory response, but in the series of Downing et al,65 LMWH was more effective than heparin in limiting neutrophil extravasation and was the only intervention to decrease vein wall permeability. In addition, heparin has been shown to prevent the accumulation of proliferating vascular smooth muscle cells encroaching on the lumen of arteries injured at angioplasty, hence enlarging the lumen.66
The mode of action of paternal leukocyte immunization (PLI) is unclear. The two most plausible explanations seem to be that PLI may downregulate the numbers and killing activity of NK cells,67 which may attack the trophoblast when activated by cytokines. PLI may also alter the balance between Th1 and Th2 cytokines. (PLI has been reported to reduce IFN-γ and increase the secretion of IL-10 and TGF-β,68 and has also been shown to lower the serum IL-6 and soluble IL-6 receptor (sIL-6R) levels to the values observed in normal pregnancy.69)
IVIG has numerous actions. The actions of IVIG in pregnancy, including its effects on APS, have been summarized elsewhere.70 In addition to various other mechanisms, IVIG, like PLI, depresses NK-cell function71 and enhances the action of Th2 cytokines.72
CONCLUSIONS
Patients with RPL seem to comprise a high-risk group for later obstetric complications. Late obstetric
complications have been described in APS and hereditary thrombophilias. However, there is insufficient evidence at present to determine whether the late obstetric complications occur exclusively in these two conditions, or whether they are associated with RPL per se. Immunomodulation seems to reduce the incidence of some of these complications. The role of anticoagulants in reducing obstetric complications is more doubtful. However, careful surveillance is required in pregnancies following RPL, in order to detect obstetric complications. Further prospective cohort studies are necessary in order more accurately define the patient at risk, the role of APS and hereditary thrombophilia, and the effect of treatment modalities.
REFERENCES
1.Reginald PW, Beard RW, Chapple J, et al. Outcome of pregnancies progressing beyond 28 weeks gestation in women with a history of recurrent miscarriage. Br J Obstet Gynaecol 1987; 94:643–8.
2.Hughes N, Hamilton EF, Tulandi T. Obstetric outcome in women after multiple spontaneous abortions. J Reprod Med 1991; 36:165–6.
3.Tulppala M, Palosuo T, Ramsay T, et al. A prospective study of 63 couples with a history of recurrent spontaneous abortion: contributing factors and outcome of subsequent pregnancies. Hum Reprod 1993; 8:764–70.
4.Jivraj S, Anstie B, Cheong YC, et al. Obstetric and neonatal outcome in women with a history of recurrent miscarriage: a cohort study. Hum Reprod 2001; 16:102–6.
5.Seidman DS, Gale R, Ever-Hadani P, et al. Reproductive complications after previous spontaneous abortions. Pediatr Rev Commun 1990; 5:1–10.
6.Sheiner E, Levy A, Katz M, et al. Pregnancy outcome following recurrent spontaneous abortions. Eur J Obstet Gynecol Reprod Biol 2005; 118:61–5.
7.Beard RW. Clinical associations of recurrent miscarriage. In: Beard RW, Sharp F, eds. Early Pregnancy Loss: Mechanisms and Treatment. London, UK: RCOG, 1988:3–8.
8. Carp HJA, Toder V, Mashiach S, et al. Recurrent miscarriage: a review of current concepts, immune mechanisms, and results of treatment. Obstet Gynecol Surv 1990; 45:657–69.
9.Recurrent Miscarriage Immunotherapy Trialists Group. Worldwide collaborative observational study and metaanalysis on allogenic leucocyte immunotherapy for recurrent spontaneous abortion. Am J Reprod Immunol 1994; 32:55–72.
10.Carp HJA, Menashe Y, Frenkel Y, et al. Lupus anticoagulant: significance in first trimester habitual abortion. J Reprod Med 1993; 38:549–52.
11.Tincani A, Balestrieri G, Danieli E, et al. Pregnancy complications of the antiphospholipid syndrome. Autoimmunity 2003; 36:27–32.
12.Brewster JA, Shaw NJ, Farquharson RG. Neonatal and pediatric outcome of infants born to mothers with antiphospholipid syndrome. J Perinat Med 1999; 27:183–7.
13.Branch DW, Dudley DJ, Scott JR, et al. Antiphospholipid antibodies and fetal loss. N Engl J Med 1992; 326:952.
239

RECURRENT PREGNANCY LOSS: CAUSES, CONTROVERSIES AND TREATMENT
14.Lima F, Khamashta MA, Buchanan NM, et al. A study of sixty pregnancies in patients with the antiphospholipid syndrome. Clin Exp Rheumatol 1996; 14:1316.
15.Branch DW, Silver RM, Blackwell JL, et al. Outcome of treated pregnancies in women with antiphospholipid syndrome: an update of the Utah experience. Obstet Gynecol 1992; 80:614–20.
16.Kutteh WH, Ermel LD. A clinical trial for the treatment of antiphospholipid antibody-associated recurrent pregnancy loss with lower dose heparin and aspirin. Am J Reprod Immunol 1996; 35:4027.
17.Pattison NS, Chamley LW, McKay EJ, et al. Antiphospholipid antibodies in pregnancy: prevalence and clinical associations. Br J Obstet Gynaecol 1993; 100: 909–13.
18.Lynch A, Marlar R, Murphy J, et al. Antiphospholipid antibodies in predicting adverse pregnancy outcome. A prospective study. Ann Intern Med 1994; 120:470–5.
19.Shehata HA, Nelson-Piercy C, Khamashta MA. Management of pregnancy in antiphospholipid syndrome. Rheum Dis Clin North Am 2001; 27:643–59.
20.Caccavo D, Vaccaro F, Ferri GM, et al. Anti-idiotypes against antiphospholipid antibodies are present in normal polyspecific immunoglobulins for therapeutic use. J Autoimmun 1994; 7:537–48.
21.Galli M, Cortelazzo S, Barbui T. In vivo efficacy of intravenous gammaglobulins in patients with lupus anticoagulant is not mediated by anti-idiotypic mechanisms. Am J Hematol 1991; 38:184–8.
22.Kwak JY, Quilty EA, Gilman-Sachs A, et al. Intravenous immunoglobulin infusion therapy in women with recurrent spontaneous abortions of immune etiologies. J Reprod Immunol 1995; 28:175–88.
23.Wegmann TG. The cytokine basis for cross-talk between the maternal immune and reproductive systems. Curr Opin Immunol 1990; 2:765–9.
24.Carreras LD, Perez GN, Vega HR, et al. Lupus anticoagulant and recurrent fetal loss: successful treatment with gammaglobulin. Lancet 1988; ii:393–4.
25.Cowchock FS, Wapner RJ, Needleman L, et al. A comparison of pregnancy outcome after two treatments for antibodies to cardiolipin (ACA). Clin Exp Rheumatol 1988; 6:200–6.
26.Triplett DA. Antiphospholipid antibodies and recurrent pregnancy loss. Am J Reprod Immunol 1989; 20:52–67.
27.Wapner RJ, Cowchock FS, Shapiro SS. Successful treatment in two women with antiphospholipid antibodies and refractory pregnancy losses with intravenous immunoglobulin infusions. Am J Obstet Gynecol 1989; 161:1271–2.
28.Vaquero E, Lazzarin N, Valensie H, et al. Pregnancy outcome in recurrent spontaneous abortion associated with antiphospholipid antibodies: a comparative study of intravenous immunoglobulin versus prednisone plus low-dose aspirin. Am J Reprod Immunol 2001; 45:174–9.
29.Branch DW, Peaceman AM, Druzin M, et al. A multicenter, placebocontrolled pilot study of intravenous immune globulin treatment of antiphospholipid syndrome during pregnancy. The Pregnancy Loss Study Group. Am J Obstet Gynecol 2000; 182:122–7.
30.Harris EN, Pierangeli SS. Utilization of intravenous immunoglobulin therapy to treat recurrent pregnancy loss in the antiphospholipid syndrome: a review. Scand J Rheumatol 1998; 107 (Suppl):97–102.
31.Kupferminc MJ, Eldor A, Steinman N, et al. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med 1999; 340:9–13.
32.Infante-Rivard C, Rivard GE, Yotov WV, et al. Absence of association of thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med 2002; 347:19–25.
33.Alfirevic Z, Roberts D, Martlew V. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? A systematic review. Eur J Obstet Gynecol Reprod Biol 2002; 101:6–14.
34.Carp HJA, Dolitzky M, Inbal A. Thromboprophylaxis improves the live birth rate in women with consecutive recurrent miscarriages and hereditary thrombophilia. J Thromb Hemost 2003; 1:433–8.
35.Younis JS, Ohel G, Brenner B, et al. Familial thrombophilia – the scientific rationale for thrombophylaxis in recurrent pregnancy loss. Hum Reprod 1997; 12:1389–90.
36.Brenner B, Hoffman R, Blumenfeld Z, et al. Gestational outcome in thrombophilic women with recurrent pregnancy loss treated by enoxaparin. Thromb Hemost 2000; 83:693–7.
37.Riyazi N, Leeda M, de Vries JI, et al. Low-molecular-weight heparin combined with aspirin in pregnant women with thrombophilia and a history of preeclampsia or fetal growth restriction: a preliminary study. Eur J Obstet Gynecol Reprod Biol 1998; 80:49–54.
38.Bates SM, Greer IA, Hirsh J, et al. Use of antithrombotic agents during pregnancy: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126:627S–44S.
39.Coulam CB, Krysa LW, Stern JJ, et al. Intravenous immunoglobulin for treatment of recurrent pregnancy loss. Am J Reprod Immunol 1995; 34:333–7.
40.Mowbray JF, Gibbings CR, Liddell H. et al. Controlled trial of treatment of recurrent spontaneous abortions by immunization with paternal cells. Lancet 1985; i:941–3.
41.Ober C, Karrison T, Odem RR, et al. Mononuclear-cell immunisation in prevention of recurrent miscarriages: a randomised trial. Lancet 1999; 354:365–9.
42.Chaouat G, Menu E, Wegmann TG. Role of lymphokines of the CSF family and of TNF, gamma interferon and IL-2 in placental growth and fetal survival studied in two murine models of spontaneous resorptions. In: Chaouat G, Mowbray JF, eds. Cellular and Molecular Biology of the Maternal–Fetal Relationship. Paris: INSERM/John Libbey Eurotext, 1991:91.
43.King A, Jokhi PP, Burrows TD, et al. Functions of human decidual NK cells. Am J Reprod Immunol 1996; 35:258–60.
44.Baines MG, Duglos AJ, de Fougerolles AR, et al. Immunological prevention of spontaneous early embryo resorption is mediated by non specific immunostimulation. Am J Reprod Immunol 1996; 35:34–42.
45. Schäfer-Somi S. Cytokines during early pregnancy of mammals: a review. Animal Reprod Sci 2003; 75:73–94.
46.Saito S. Cytokine network at the feto–maternal interface. J Reprod Immunol 2000; 47:87–103.
47.Savion S, Brengauz-Breitmann M, Torchinsky A, et al. A possible role for granulocyte macrophage-colony stimulating factor in modulating teratogen-induced effects. Teratog Carcinog Mutagen 1999; 19:171–82.
48.Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med 1999; 341:586–92.
49.Bertolaccini ML, Atsumi T, Lanchbury JS, et al. Plasma tumor necrosis factor α levels and the 238*A promoter polymorphism in
patients with antiphospholipid syndrome. Thromb Haemost 2001; 85:198–203.
50.Shoenfeld Y, Sherer Y, Fishman P. Interleukin-3 and pregnancy loss in antiphospholipid syndrome. Scand J Rheumatol 1998; 107 (Suppl): 19–22.
51.Fishman P, Falach-Vaknine E, Zigelman R, et al. Prevention of fetal loss in experimental antiphospholipid syndrome by in vivo administration of recombinant interleukin-3. J Clin Invest 1993; 91:1834–7.
52.Krause I, Blank M, Levi Y, et al. Anti-idiotype immunomodulation of experimental anti-phospholipid syndrome via effect on Th1/Th2 expression. Clin Exp Immunol 1999; 117:190–7.
240

OBSTETRIC OUTCOMES AFTER RECURRENT PREGNANCY LOSS
53.Darmochwal-Kolarz D, Rolinski J, Leszczynska-Goarzelak B, et al. The expressions of intracellular cytokines in the lymphocytes of preeclamptic patients. Am J Reprod Immunol 2002; 48:381.
54.Orange S, Horvath J, Hennessy A. Preeclampsia is associated with a reduced interleukin-10 production from peripheral blood mononuclear cells. Hypertens Pregnancy 2003; 22:1–8.
55.Velzing-Aarts FV, Muskiet FA, Van der Dijs FP, et al. High serum
interleukin-8 levels in Afro-Caribbean women with pre-eclampsia. Relations with tumor necrosis factor-α, Duffy negative phenotype
and von Willebrand factor. Am J Reprod Immunol 2002; 48:319–22.
56.Park JS, Park CW, Lockwood CJ, et al. Role of cytokines in preterm labor and birth. Minerva Ginecol 2005; 57:349–66.
57.Fortunato SJ, Menon R, Lombardi SJ. Role of tumor necrosis factor-α in the premature rupture of membranes and preterm labor pathways. Am J Obstet Gynecol 2002; 187:1159–62.
58.Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand 2003; 82:120–8.
59.Maymon E, Ghezzi F, Edwin SS, et al. The tumor necrosis factor-α and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol 1999; 181:1142–8.
60.Ognjanovic S, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am J Obstet Gynecol 2002; 187:1051–8.
61.Hahn-Zoric M, Hagberg H, Kjellmer I, et al. Aberrations in placental cytokine mRNA related to intrauterine growth retardation. Pediatr Res 2002; 51:201–6.
62.Holcberg G, Huleihel M, Sapir O, et al. Increased production of tumor necrosis factor-α (TNF-α) by IUGR human placentae. Eur J Obstet
Gynecol Reprod Biol 2001; 94:69–72.
63.Lantz M, Thysell H, Nilsson E, et al. On the binding of tumor necrosis factor (TNF) to heparin and the release in vivo of the TNF-binding protein I by heparin. J Clin Invest 1991; 88:2026–31.
64.Baram D, Rashkovsky M, Hershkoviz R, et al. Inhibitory effects of low molecular weight heparin on mediator release by mast cells: preferential inhibition of cytokine production and mast cell-dependent cutaneous inflammation. Clin Exp Immunol 1997; 110:485–91.
65.Downing LJ, Strieter RM, Kadell AM, et al. Low-dose low-molecular-
weight heparin is anti-inflammatory during venous thrombosis. J Vasc Surg 1998; 28:848–54.
66.San Antonio JD, Verrecchio A, Pukac LA. Heparin sensitive and resistant vascular smooth muscle cells: biology and role in restenosis. Conn Tiss Res 1998; 37:87–103.
67.Kwak JY, Gilman-Sachs A, Moretti M, et al. Natural killer cell cytotoxicity and paternal lymphocyte immunization in women with recurrent spontaneous abortions. Am J Reprod Immunol 1998; 40:352–8.
68.Gafter U, Sredni B, Segal J, et al. Suppressed cell-mediated immunity and monocyte and natural killer cell activity following allogeneic immunization of women with spontaneous recurrent abortion. J Clin Immunol 1997; 17:408–19.
69.Zenclussen AC, Kortebani G, Mazzolli A, et al. Interleukin-6 and soluble interleukin-6 receptor serum levels in recurrent spontaneous abortion women immunized with paternal white cells. Am J Reprod Immunol 2000; 44:22–9.
70.Carp HJA, Sapir T, Shoenfeld Y. Intravenous immunoglobulin and recurrent pregnancy loss. Clin Rev Allergy Immunol 2005; 29:327–32.
71.Ruiz JE, Kwak JY, Baum L, et al. Effect of intravenous immunoglobulin G on natural killer cell cytotoxicity in vitro in women with recurrent spontaneous abortion. J Reprod Immunol 1996; 31:125–41.
72.Graphou O, Chioti A, Pantazi A, et al. Effect of intravenous immunoglobulin treatment on the Th1/Th2 balance in women with recurrent spontaneous abortions. Am J Reprod Immunol 2003; 49:21–9.
241

