
- •Overview
- •Preface
- •Translator’s Note
- •Contents
- •1. Fundamentals
- •Microscopic Anatomy of the Nervous System
- •Elements of Neurophysiology
- •Elements of Neurogenetics
- •General Genetics
- •Neurogenetics
- •Genetic Counseling
- •2. The Clinical Interview in Neurology
- •General Principles of History Taking
- •Special Aspects of History Taking
- •3. The Neurological Examination
- •Basic Principles of the Neurological Examination
- •Stance and Gait
- •Examination of the Head and Cranial Nerves
- •Head and Cervical Spine
- •Cranial Nerves
- •Examination of the Upper Limbs
- •Motor Function and Coordination
- •Muscle Tone and Strength
- •Reflexes
- •Sensation
- •Examination of the Trunk
- •Examination of the Lower Limbs
- •Coordination and Strength
- •Reflexes
- •Sensation
- •Examination of the Autonomic Nervous System
- •Neurologically Relevant Aspects of the General Physical Examination
- •Neuropsychological and Psychiatric Examination
- •Psychopathological Findings
- •Neuropsychological Examination
- •Special Considerations in the Neurological Examination of Infants and Young Children
- •Reflexes
- •4. Ancillary Tests in Neurology
- •Fundamentals
- •Imaging Studies
- •Conventional Skeletal Radiographs
- •Computed Tomography (CT)
- •Magnetic Resonance Imaging (MRI)
- •Angiography with Radiological Contrast Media
- •Myelography and Radiculography
- •Electrophysiological Studies
- •Fundamentals
- •Electroencephalography (EEG)
- •Evoked potentials
- •Electromyography
- •Electroneurography
- •Other Electrophysiological Studies
- •Ultrasonography
- •Other Ancillary Studies
- •Cerebrospinal Fluid Studies
- •Tissue Biopsies
- •Perimetry
- •5. Topical Diagnosis and Differential Diagnosis of Neurological Syndromes
- •Fundamentals
- •Muscle Weakness and Other Motor Disturbances
- •Sensory Disturbances
- •Anatomical Substrate of Sensation
- •Disturbances of Consciousness
- •Dysfunction of Specific Areas of the Brain
- •Thalamic Syndromes
- •Brainstem Syndromes
- •Cerebellar Syndromes
- •6. Diseases of the Brain and Meninges
- •Congenital and Perinatally Acquired Diseases of the Brain
- •Fundamentals
- •Special Clinical Forms
- •Traumatic Brain injury
- •Fundamentals
- •Traumatic Hematomas
- •Complications of Traumatic Brain Injury
- •Intracranial Pressure and Brain Tumors
- •Intracranial Pressure
- •Brain Tumors
- •Cerebral Ischemia
- •Nontraumatic Intracranial Hemorrhage
- •Infectious Diseases of the Brain and Meninges
- •Infections Mainly Involving the Meninges
- •Infections Mainly Involving the Brain
- •Intracranial Abscesses
- •Congenital Metabolic Disorders
- •Acquired Metabolic Disorders
- •Diseases of the Basal Ganglia
- •Fundamentals
- •Diseases Causing Hyperkinesia
- •Other Types of Involuntary Movement
- •Cerebellar Diseases
- •Dementing Diseases
- •The Dementia Syndrome
- •Vascular Dementia
- •7. Diseases of the Spinal Cord
- •Anatomical Fundamentals
- •The Main Spinal Cord Syndromes and Their Anatomical Localization
- •Spinal Cord Trauma
- •Spinal Cord Compression
- •Spinal Cord Tumors
- •Myelopathy Due to Cervical Spondylosis
- •Circulatory Disorders of the Spinal Cord
- •Blood Supply of the Spinal Cord
- •Arterial Hypoperfusion
- •Impaired Venous Drainage
- •Infectious and Inflammatory Diseases of the Spinal Cord
- •Syringomyelia and Syringobulbia
- •Diseases Mainly Affecting the Long Tracts of the Spinal Cord
- •Diseases of the Anterior Horns
- •8. Multiple Sclerosis and Other Myelinopathies
- •Fundamentals
- •Myelin
- •Multiple Sclerosis
- •Other Demyelinating Diseases of Unknown Pathogenesis
- •9. Epilepsy and Its Differential Diagnosis
- •Types of Epilepsy
- •Classification of the Epilepsies
- •Generalized Seizures
- •Partial (Focal) Seizures
- •Status Epilepticus
- •Episodic Neurological Disturbances of Nonepileptic Origin
- •Episodic Disturbances with Transient Loss of Consciousness and Falling
- •Episodic Loss of Consciousness without Falling
- •Episodic Movement Disorders without Loss of Consciousness
- •10. Polyradiculopathy and Polyneuropathy
- •Fundamentals
- •Polyradiculitis
- •Cranial Polyradiculitis
- •Polyradiculitis of the Cauda Equina
- •Polyneuropathy
- •Fundamentals
- •11. Diseases of the Cranial Nerves
- •Fundamentals
- •Disturbances of Smell (Olfactory Nerve)
- •Neurological Disturbances of Vision (Optic Nerve)
- •Visual Field Defects
- •Impairment of Visual Acuity
- •Pathological Findings of the Optic Disc
- •Disturbances of Ocular and Pupillary Motility
- •Fundamentals of Eye Movements
- •Oculomotor Disturbances
- •Supranuclear Oculomotor Disturbances
- •Lesions of the Nerves to the Eye Muscles and Their Brainstem Nuclei
- •Ptosis
- •Pupillary Disturbances
- •Lesions of the Trigeminal Nerve
- •Lesions of the Facial Nerve
- •Disturbances of Hearing and Balance; Vertigo
- •Neurological Disturbances of Hearing
- •Disequilibrium and Vertigo
- •The Lower Cranial Nerves
- •Accessory Nerve Palsy
- •Hypoglossal Nerve Palsy
- •Multiple Cranial Nerve Deficits
- •12. Diseases of the Spinal Nerve Roots and Peripheral Nerves
- •Fundamentals
- •Spinal Radicular Syndromes
- •Peripheral Nerve Lesions
- •Fundamentals
- •Diseases of the Brachial Plexus
- •Diseases of the Nerves of the Trunk
- •13. Painful Syndromes
- •Fundamentals
- •Painful Syndromes of the Head And Neck
- •IHS Classification of Headache
- •Approach to the Patient with Headache
- •Migraine
- •Cluster Headache
- •Tension-type Headache
- •Rare Varieties of Primary headache
- •Symptomatic Headache
- •Painful Syndromes of the Face
- •Dangerous Types of Headache
- •“Genuine” Neuralgias in the Face
- •Painful Shoulder−Arm Syndromes (SAS)
- •Neurogenic Arm Pain
- •Vasogenic Arm Pain
- •“Arm Pain of Overuse”
- •Other Types of Arm Pain
- •Pain in the Trunk and Back
- •Thoracic and Abdominal Wall Pain
- •Back Pain
- •Groin Pain
- •Leg Pain
- •Pseudoradicular Pain
- •14. Diseases of Muscle (Myopathies)
- •Structure and Function of Muscle
- •General Symptomatology, Evaluation, and Classification of Muscle Diseases
- •Muscular Dystrophies
- •Autosomal Muscular Dystrophies
- •Myotonic Syndromes and Periodic Paralysis Syndromes
- •Rarer Types of Muscular Dystrophy
- •Diseases Mainly Causing Myotonia
- •Metabolic Myopathies
- •Acute Rhabdomyolysis
- •Mitochondrial Encephalomyopathies
- •Myositis
- •Other Diseases Affecting Muscle
- •Myopathies Due to Systemic Disease
- •Congenital Myopathies
- •Disturbances of Neuromuscular Transmission−Myasthenic Syndromes
- •15. Diseases of the Autonomic Nervous System
- •Anatomy
- •Normal and Pathological Function of the Autonomic Nervous System
- •Sweating
- •Bladder, Bowel, and Sexual Function
- •Generalized Autonomic Dysfunction
- •Index

98 6 Diseases of the Brain and Meninges
and cavernoma. Craniopharyngioma arises in or above the pituitary fossa, often growing upward toward the diencephalon and third ventricle. This is a cystic tumor derived from epithelial remnants in Rathke’s pouch, generally containing calcifications as well as cholesterol crystals. It presents with hypopituitarism (see above), diencephalic manifestations (diabetes insipidus), and visual disturbances. Like a pituitary tumor, it can cause hemior quadrantanopsia and impair visual acuity; it can also cause occlusive hydrocephalus. Craniopharyngioma is the most common suprasellar tumor in children and adolescents. It is best treated by complete resection.
Cavernoma (also called cavernous angioma or cavernous malformation) consists of a well-demarcated agglomeration of blood vessels. Cavernomas can be multiple and familial (genetic locus on chromosome 7). They present with epileptic seizures and hemorrhage.
Epidermoid tumors are found at the base of the brain, are often calcified, and cause focal deficits or epileptic seizures. Their peak incidence is between the ages of 25 and 45.
Neurinomas (schwannomas) are benign neoplasms arising from Schwann cells. The most common type affects the eighth cranial nerve and is usually (though incorrectly) designated acoustic neuroma. This tumor of the cerebellopontine angle presents initially with eighth nerve dysfunction: progressive hearing loss, tinnitus, and disequilibrium. As it grows, it impinges on the other cranial nerves of the cerebellopontine angle, causing fa-
cial palsy and trigeminal sensory deficits. Further growth leads to compression of the cerebellum and brainstem, causing cerebellar signs (esp. ataxia) and possibly pyramidal tract signs. Acoustic neuroma typically markedly elevates the CSF protein concentration. Until recently, the optimal treatment in all patients was complete resection of the tumor. Now many smaller acoustic neuromas can be treated safely and effectively with stereotactic radiosurgery.
Brain metastases account for about 15 % of malignant brain tumors. The most common source of a brain metastasis is bronchial carcinoma in men and carcinoma of the breast in women, followed in both sexes by melanoma and renal cell carcinoma. Brain metastases sometimes produce symptoms before the primary tumor does; in such cases, multiple brain metastases are usually already present, even if only a single one is apparent on the neuroimaging study. Generally speaking, surgical resection makes sense only for solitary metastases and the surgical indication should always be carefully considered in the light of the extent of disease. Only about 20 % of patients so treated live more than five years after the operation and postoperative radiotherapy, if they have not already died of the effects of their primary tumor. Brain metastases usually produce extensive peritumoral edema and often cause epileptic seizures; thus, corticosteroids and antiepileptic drugs can be given for palliation. This usually brings a substantial, if only temporary, clinical improvement.
Circulatory Disorders of the Brain
and Nontraumatic Intracranial Hemorrhage
The term “stroke” encompasses both ischemic and hemorrhagic disturbances of the cerebral circulation producing central neurological deficits of acute or subacute onset. Ischemia accounts for 80 to 85 % of stroke, hemorrhage for 15 to 20 %.
Cerebral Ischemia
Ischemia causes critical hypoperfusion in an area of the brain. Depending on its extent and duration, hypoperfusion can induce neurological deficits that may be either transient (TIA, RIND) or permanent (completed stroke, infarction). The more common causes of ischemia are blockage of the arterial blood supply by arteriosclerotic processes of both larger and smaller blood vessels (macroangiopathic and microangiopathic processes) and embolic events (arterio-arterial and cardiogenic embolization). A less common cause is obstruction of venous outflow (e. g., venous sinus thrombosis). Every ischemic event should prompt thorough diagnostic evaluation to identify its etiology, so that effective measures can be taken to prevent a recurrence.
Nontraumatic Intracranial Hemorrhage
Regulation of cerebral perfusion. Glucose is the brain’s nearly exclusive source of energy. The brain accounts for only about 2 % of body weight, but it receives about 15 % of the cardiac output. Regulatory mechanisms ensure that the cerebral perfusion remains constant despite fluctuations in the arterial blood pressure, as long as the latter remains within a certain range. Thus, if the arterial blood pressure should fall, a compensatory dilatation of the cerebral arteries occurs to maintain cerebral perfusion, which is significantly reduced only when the systolic blood pressure falls below 70 mmHg (or below 70 % of the baseline value in hypertensive individuals). Hyperventilation and elevated intracranial pressure reduce cerebral perfusion, while hypoventilation (i. e., an elevated partial pressure of CO2) increases it.
Consequences of ischemia. Normal cerebral perfusion is ca. 58 mL per 100 g brain tissue per minute. Signs and symptoms of ischemia begin to appear when perfusion falls below 22 mL per 100 g per min. In this stage of relative ischemia, the functional metabolism of the affected brain tissue is impaired, but the infarction threshold has not yet been reached and the tissue can regain its normal function as soon as the perfusion renormalizes. The longer relative ischemia lasts, however, the less likely it
Mumenthaler / Mattle, Fundamentals of Neurology © 2006 Thieme All rights reserved. Usage subject to terms and conditions of license.
Circulatory Disorders of the Brain and Nontraumatic Intracranial Hemorrhage 99
is that normal function will be regained. The zone of tissue in which the local cerebral perfusion lies between the functional threshold and the infarction threshold is called the ischemic penumbra (“partial shadow”).
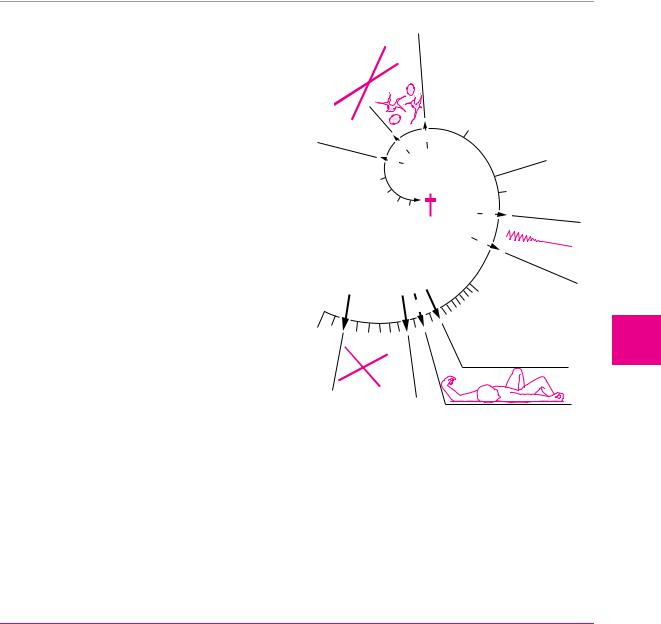
Total ischemia causes irreversible structural damage of the affected region of the brain. If the blood supply of the entire brain is cut off, unconsciousness ensues in 10 to 12 seconds and cerebral electrical activity, as demonstrated by EEG, ceases in 30 to 40 seconds (Fig. 6.9). Cellular metabolism collapses, the sodium/potassium pump ceases to function, and interstitial fluid—i. e., sodium and water—flows into the cells. The resulting cellular swelling is called cytotoxic cerebral edema. Later, when the blood−CSF barrier collapses, further plasma components, including osmotically active substances, enter the brain tissue; a net flow of fluid from the intravascular space into the intercellular and intracellular spaces then produces vasogenic cerebral edema. In a vicious circle, these two varieties of edema lead to additional compression of brain tissue, thereby impairing the cerebral perfusion still further.
Dynamic time course of cerebral ischemia. Cerebral perfusion can cause a wide variety of clinical manifestations. In clinical practice, these are often classified by their temporal course and their degree of reversibility or irreversibility (Table 6.11). Although classification in this way is useful, it says nothing about the underlying etiology of the ischemic events. Moreover, the boundaries between the listed entities (e. g., TIA and RIND) are not sharp.
Arterial blood supply of the brain. To understand how the localization and extent of cerebral infarcts depends on the particular artery that is occluded, one must know the anatomy of the territories of the individual vessels, as well as their numerous anastomoses. The anastomotic arterial circle of Willis, at the base of the brain, provides a connection between the carotid and verte-
|
|
|
E |
|
|
|
|
|
|
|
S |
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
L |
U |
C |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
G |
|
|
|
|
3 |
|
|
|
|
|
|
4 |
2 |
|
|
||
|
|
|
|
|
|
|||
|
|
|
5 |
|
|
|
minutes |
|
|
|
|
|
|
|
|
||
|
|
6 |
|
|
|
|
||
|
|
|
|
|
1 |
seconds |
||
|
|
|
7 |
|
9 |
|
||
|
|
|
|
|
|
|||
|
|
|
8 |
|
|
50 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
40 |
|
|
|
|
|
|
|
|
30 |
EEG |
|
|
|
|
|
|
|
|
|
0 |
|
|
|
|
|
20 |
|
|
|
|
|
10 |
|
|
|
||
|
|
|
|
|
|
|
||
|
O2 |
|
|
|
|
|
|
|
Fig. 6.9 Time course of cerebral ischemia. Diagram of the effect of sudden total deprivation of blood supply to the brain on tissue metabolism, consciousness, the EEG, neuronal morphology, and tissue glucose concentration.
bral circulations and between the blood supplies of the right and left cerebral hemispheres (Fig. 6.10). The territories of the major cerebral arteries are shown in Fig. 6.11.
Table 6.11 Classification of cerebral ischemia by temporal course
Designation |
Duration of deficits |
Remarks |
|
|
|
TIA = transient ischemic attack |
usually 2−15 minutes, sometimes as long as 24 |
transient focal neurological and/or neu- |
|
hours |
ropsychological deficit (e. g., aphasia); a |
|
|
TIA in the distribution of the ophthalmic |
|
|
a. presents as amaurosis fugax |
RIND = reversible ischemic neuro- |
up to 7 days |
mostly minor neurological deficits |
logical deficit |
|
|
stroke in evolution, progressive |
stroke with neurological deficits that continue to |
|
stroke |
worsen for hours or days after onset |
|
completed stroke |
established neurological deficit that is irreversible |
|
|
or only partly reversible |
|
|
|
|
blubber blubber
Mumenthaler / Mattle, Fundamentals of Neurology © 2006 Thieme All rights reserved. Usage subject to terms and conditions of license.
6
Diseases of the Brain and Meninges

100 6 Diseases of the Brain and Meninges
Fig. 6.10 Arteries of the base of the skull (after Baehr M. and M. Frotscher: Duus’ Topical Diagnosis in Neurology, 4th edn, Thieme, Stuttgart 2005).
anterior communicating a.
anterior cerebral a. internal carotid a. middle cerebral a. posterior communicating a. posterior cerebral a.
anterior choroidal a.
superior cerebellar a. basilar a.
anterior inferior cerebellar a. labyrinthine a.
posterior inferior cerebellar a.
vertebral a.
anterior spinal a.
|
thalamic aa. |
|
|
posterior cerebral a. |
|
lenticulostriate aa. |
superior cerebellar a. |
|
anterior inferior |
||
middle cerebral a. |
cerebellar a. |
|
anterior cerebral a. |
posterior inferior |
|
anterior choroidal a. |
cerebellar a. |
|
vertebral aa./ |
||
|
||
|
basilar a. |
|
|
vertebrobasilar |
|
carotid circulation |
circulation |
Fig. 6.11 Territories supplied by the individual arteries of the brain.
Mumenthaler / Mattle, Fundamentals of Neurology © 2006 Thieme
All rights reserved. Usage subject to terms and conditions of license.

Circulatory Disorders of the Brain and Nontraumatic Intracranial Hemorrhage
Ischemic Stroke
Ischemic stroke occurs when persistent ischemia or a complete interruption of the blood supply to a particular area of the brain produces irreversible destruction of brain tissue. The resulting neurological deficits usually arise quite suddenly (whence the term “stroke”) but can sometimes progress over a longer period of time (“stroke in evolution”). They are irreversible, or at most only partly reversible.
Table 6.12 Classification of ischemic stroke by etiology
I.Atherosclerosis of large extraand intracranial vessels, leading to:
thrombosis in the region of an atherosclerotic plaque,
hemodynamic insufficiency in the poststenotic circulation, or
arterio-arterial embolism
II.Cardiogenic and aortogenic embolism
III.Cerebral small vessel disease/arteriolosclerosis, usually due to hypertension
IV. Other etiologies, e. g.:
vasculopathies
coagulopathies
Etiology. Ischemic stroke has many causes. Embolic events and vascular stenosis due to atherosclerosis play important roles, as do hypertensive atherosclerotic changes of medium-caliber or small cerebral arteries.
!The most important risk factor for stroke is arterial hypertension.
The major etiologies of ischemic stroke are summarized in Table 6.12. The acute symptoms are sometimes produced by a sudden drop in blood pressure. The major risk factors for atherosclerosis and ischemic stroke are listed in Table 6.13.
Types of infarct. There are three basic types of brain infarct, distinguished from each other by the caliber of the occluded arteries:
V.Undetermined etiology
Table 6.13 Risk factors for atherosclerosis and ischemic stroke
Positive family history of early onset of atherosclerotic disease ( 55 years of age)
Arterial hypertension
Cigarette smoking
Truncal obesity, hypercholesterolemia
Diabetes mellitus
Sleep apnea syndrome
Past history of cardioor cerebrovascular disease or occlusive peripheral vascular disease
Territorial infarcts are mainly produced by occlusions of the main trunks or major branches of cerebral arteries
(cerebral macroangiopathy), which may be due to thrombosis, embolism, or other causes. The infarct includes both cortex and subcortical white matter and sometimes the basal ganglia and thalamus (Fig. 6.12). It is usually possible to infer which vessel has been occluded from the pattern of neurological deficits that are produced.
Watershed infarcts (border zone infarcts) are infarcts of hemodynamic origin that are likewise due to microangiopathic processes. Narrowing of small vessels impairs perfusion in the vulnerable regions at the borders between the territories of two or more arteries (Fig. 6.13). If the perfusion pressure is inadequate, infarction ensues.
a |
b |
Fig. 6.12 Infarct in the territory of the left middle cerebral a. in a 60-year-old man with acute right hemiplegia. a The left carotid angiogram (a−p view) reveals occlusion of the main stem of the middle cerebral a. at its origin. Only the anterior cerebral artery is visualized. b The lateral view shows only the pericallosal artery, with
blubber blubber
c
its branches, and the posterior cerebral a., while the middle cerebral a. and its branches are not seen (cf. normal carotid angiogram, p. 51). c A CT scan obtained 2 days after the onset of symptoms reveals massive infarction in the territory of the middle cerebral a., extending from the cortex to the basal ganglia.
Mumenthaler / Mattle, Fundamentals of Neurology © 2006 Thieme All rights reserved. Usage subject to terms and conditions of license.
101
6
Diseases of the Brain and Meninges
102 6 Diseases of the Brain and Meninges
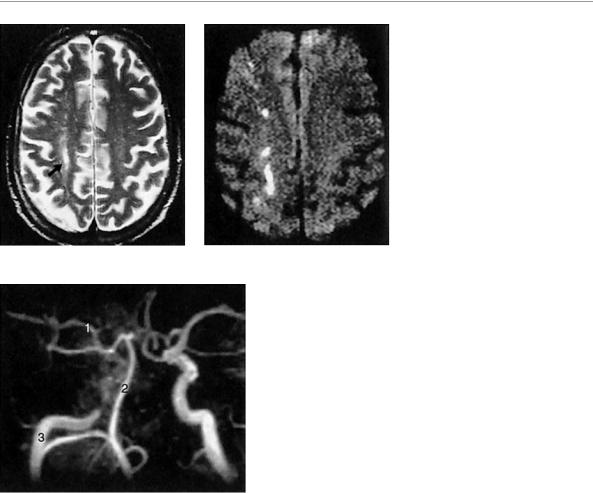
Fig. 6.13 Watershed infarct. A 58-year- old man with an infarct in the watershed zone between the right anterior and middle cerebral a. territories, caused by occlusion of the right internal carotid a. The vascular territories are shown in Fig. 6.10 (p. 100). The infarct is well seen in the T2-weighted spin-echo-image (arrow in a), and still better in the diffu-
sion-weighted image (b), as a longitudinal signal abnormality in the watershed zone between the two arterial territories. The internal cerebral a. is occluded below the siphon, as the MR angiogram shows (c). 1, middle cerebral a.; 2, basilar a.; 3, right internal carotid a.
a |
b |
c
Lacunar infarcts are caused by microangiopathy (usually atherosclerosis of small vessels due to hypertension). The infarcts (lacunes) are less than 1.5 cm in diameter and often multiple. They are found mainly in the basal ganglia, thalamus, and brainstem, and sometimes in the cerebral cortex and subcortical white matter (Fig. 6.13). Their clinical presentation depends on their number and localization. Multiple subcortical infarcts due to hypertension are the hallmark of subcortical arteriosclerotic encephalopathy, also called Binswanger disease (Fig. 6.14).
Signs and Symptoms of Ischemic Stroke. The neurological deficits produced by ischemic stroke depend on the area of the brain that is ischemic or infarcted. We will now briefly summarize the clinical manifestations of the major cerebrovascular syndromes and the typical deficits produced by ischemia in circumscribed areas of the brain.
Ophthalmic a. Transient ischemia in the territory of this vessel produces amaurosis fugax (transient monocular blindness), while longer-lasting ischemia causes retinal infarction. Retinal ischemia is often due to embolism of cholesterol crystals from ulcerating plaques in
the internal carotid a. into the ophthalmic a. Embolized crystals within the arteries of the retina can occasionally be seen by ophthalmoscopy.
Internal carotid a. Stenosis or occlusion of this vessel can cause simultaneous ischemia of the eye with monocular visual loss (see above) and contralateral hemiparesis, in combination with neuropsychological deficits. This oculocerebral syndrome is rare, however, as ischemia in the territory of the internal carotid a. usually presents with either monocular visual loss or variably severe hemiparesis and neuropsychological deficits.
Middle cerebral a. (MCA). The site of occlusion (main trunk vs. branch of the middle cerebral a.) determines the clinical manifestations. As a rule, a mainly brachiofacial hemiparesis and hemisensory deficit are found, often accompanied by homonymous hemior quadrantanopsia and, in the initial phase, a horizontal gaze palsy toward the side of the hemiparesis. An MCA occlusion on the language-dominant (usually left) side additionally produces aphasia and apraxia, while one on the nondominant side produces impairment of spatial orientation. An occlusion of the main stem of the MCA causes ischemia not only of the cortex, but also of the basal
Mumenthaler / Mattle, Fundamentals of Neurology © 2006 Thieme All rights reserved. Usage subject to terms and conditions of license.
Circulatory Disorders of the Brain and Nontraumatic Intracranial Hemorrhage 103
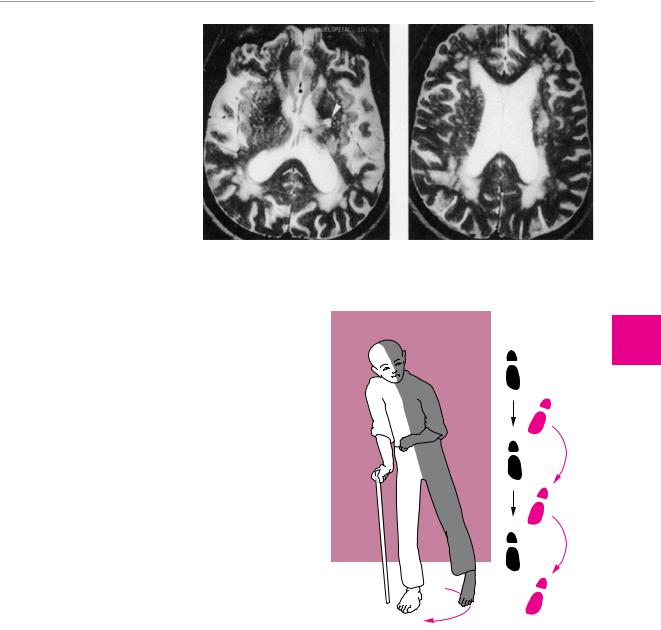
Fig. 6.14 Binswanger disease (cerebral microangiopathy) with lacunar infarct (arrowhead) and severe white matter changes. MR images in a 70-year-old man. The microangiopathic lesions are seen on the T2-weighted images as multifocal signal abnormalities in the white matter. The most severe changes are typically found in the periventricular zones abutting the frontal and occipital horns of the lateral ventricles.
ganglia and internal capsule, producing a more severe contralateral hemiparesis. If the hemiparesis fails to improve over time, or does so only partially, a typical, permanent impairment of gait results: circumduction of the spastically extended lower limb, flexion of the paretic upper limb at the wrist and elbow, and absence of arm swing on the affected side (Wernicke−Mann gait) (Fig. 6.15).
Anterior choroidal a. Ischemia in the territory of this vessel causes a homonymous visual field defect, a hemisensory deficit, and, less commonly, hemiparesis. The clinical manifestations resemble those of occlusion of the lenticulostriate aa. (branches of the middle cerebral a. supplying the basal ganglia and internal capsule). There may also be extrapyramidal motor signs, such as hemiballism.
Anterior cerebral a. An infarct in the territory of this artery causes contralateral hemiparesis mainly affecting the lower limb, sometimes accompanied by contralateral ataxia and, if the lesion is left-sided, by apraxia. Occasionally there may be apathy, abulia (pathological lack of drive and motivation), and urinary incontinence.
Posterior cerebral a. Occlusion of this artery can produce infarction in the cerebral peduncle, the thalamus, mediobasal portions of the temporal lobe, and the occipital lobe. The most prominent clinical sign of a distal occlusion (beyond the origin of the posterior communicating a.) is contralateral homonymous hemianopsia, possibly combined with neuropsychological deficits.
Basilar a. Occlusion of the main stem or of a branch of the basilar a. causes brainstem, cerebellar, and thalamic signs (see below). Main stem thrombosis can produce locked-in syndrome and is often fatal (p. 77).
Thalamic infarction results from occlusion of one of the arteries supplying the thalamus. It usually presents with a contralateral hemisensory deficit, in addition to mild paresis and hemiataxia. The patient’s memory, too, is often impaired.
Fig. 6.15 Typical gait disturbance of a hemiplegic patient. Circumduction of the spastically paretic leg with predominant extensor tone, and flexion of the spastically paretic arm at the elbow because of predominant flexor tone.
Brainstem infarcts are usually lacunar. They arise in the territory of one or more small perforating arteries that branch off the basilar trunk. Their clinical presentation depends on the particular brainstem nuclei and fiber tracts that they affect. Brainstem stroke therefore takes many different clinical forms, corresponding to the wide variety of functions served by brainstem structures. As a rule, brainstem stroke causes ipsilateral cranial nerve deficits and a contralateral hemisensory defect and/or hemiparesis (cf. Table 6.14).
The large number of brainstem vascular syndromes that have been described and given eponymous names are only rarely seen in “pure” form in clinical practice.
blubber blubber
Mumenthaler / Mattle, Fundamentals of Neurology © 2006 Thieme All rights reserved. Usage subject to terms and conditions of license.
6
Diseases of the Brain and Meninges

104 |
6 Diseases of the Brain and Meninges |
|
|
|
||
|
|
|
|
|
|
|
|
|
Table 6.14 Selected brainstem syndromes |
|
|
|
|
|
|
|
|
|
|
|
|
|
Name |
Localization |
Ipsilateral signs |
Contralateral signs |
Special features |
|
|
|
|
|
|
|
|
|
Benedikt syndrome |
midbrain, red nucleus |
oculomotor palsy, |
hemiataxia (someti- |
staggering gait |
|
|
(upper red nucleus |
|
sometimes gaze palsy |
mes), intention tremor, |
|
|
|
syndrome) |
|
toward the side of the |
hemiparesis (often |
|
|
|
|
|
lesion |
without Babinski sign) |
|
|
|
Weber syndrome |
midbrain, cerebral |
oculomotor nerve palsy |
hemiparesis |
|
|
|
|
peduncle |
|
|
|
|
|
Millard−Gubler |
posterior portion of |
peripheral facial palsy |
hemiparesis |
|
|
|
syndrome |
pontine tegmentum |
|
|
|
|
|
Wallenberg syndrome |
dorsolateral medulla |
Horner syndrome, vocal |
dissociated sensory |
nystagmus (this syn- |
|
|
|
|
cord paresis, palatal |
disturbance |
drome is caused by |
|
|
|
|
and posterior pharyn- |
|
occlusion of the poste- |
|
|
|
|
geal paresis, trigeminal |
|
rior inferior cerebellar |
|
|
|
|
nerve deficit, hemi- |
|
a.) |
|
|
|
|
ataxia |
|
|
|
|
|
|
|
|
|
Fig. 6.16 Acute infarct in the left cerebellar hemisphere, revealed by MRI. The infarct involves the territory of the superior cerebellar a. There are also two small ischemic foci in the left cerebral hemisphere.
The most important among them is Wallenberg syndrome, which results from occlusion of the posterior inferior cerebellar a. (PICA). Some of the more common vascular syndromes affecting different parts of the brainstem are summarized in Table 6.14.
Cerebellar infarction presents with vertigo, nausea, unsteady gait, dysarthria, and often acute headache and progressive impairment of consciousness. The neurological examination reveals ataxia, dysmetria, and nystagmus. Often, simultaneous infarction of part of the brainstem produces additional brainstem signs. Not uncommonly, edema in and around the infarcted area rapidly leads to a life-threatening elevation of intracranial pressure in the posterior fossa. A typical MR image of cerebellar stroke is presented in Fig. 6.16.
Diagnostic Evaluation of Ischemic Stroke. Diagnostic evaluation in the acute phase is focused on the determination of the anatomic site and extent of cerebral ischemia and, above all, its etiology.
Acute diagnostic evaluation. In pursuit of these goals, the initial work-up should always begin with the following:
precise history taking concerning not only the present illness, but also the past medical history, with special attention to risk factors and systemic illnesses;
a thorough clinical neurological examination enabling localization of the lesion (see above); and
examination of the cardiovascular system (measurement of pulse and blood pressure and auscultation of the heart, the carotid aa., and perhaps other vessels, depending on the clinical situation; particular attention should be paid to bruits and to any irregularities of the pulse that suggest arrhythmia).
Ancillary testing in the acute phase. The following ancillary tests should also be performed on all stroke patients in the acute phase:
Laboratory tests, mainly for the identification of risk factors, infectious/inflammatory disorders, and coagulopathies (erythrocyte sedimentation rate, blood sugar, lipid profile, complete blood count and hemoglobin, coagulation profile, and sometimes protein C, antiphospholipid antibodies, syphilis serology, etc.).
Imaging studies. Even before these are performed, any central neurological deficit of acute onset is very likely to be due to a cerebrovascular accident, of which ischemic stroke is the most common type; yet neuroimaging is still indicated for definitive confirmation of the diagnosis. Any patient thought to be suffering from acute ischemic stroke should undergo CT as soon as possible, as this will have important implications for the course of treatment, even though areas of ischemia usually cannot be seen by CT till several hours after the onset of symptoms. Early CT does, however, reveal acute brain hemorrhage, if present. MRI can also be performed, if avail-
Mumenthaler / Mattle, Fundamentals of Neurology © 2006 Thieme All rights reserved. Usage subject to terms and conditions of license.
Circulatory Disorders of the Brain and Nontraumatic Intracranial Hemorrhage 105
able. MRI reveals the infarct zone and perifocal edema as soon as the patient begins to experience symptoms and it displays brainstem and cerebellar infarcts more clearly than CT.
Doppler ultrasonography of the extraand intracranial vessels to detect vascular stenosis, occlusion, and vascular collateralization.
An electrocardiogram (arrhythmia pointing to a likely cardioembolic event? Old or acute myocardial infarction? Evidence of regional cardiac wall motion abnormalities, creating a danger of intracardiac thrombosis and embolism?).
!When an ischemic stroke is suspected, the most important immediate question in the differential diagnosis is whether the patient is not, in fact, suffering from an intracerebral hemorrhage, rather than from cerebral ischemia. The history and physical examination alone cannot provide a reliable answer; therefore, a neuroimaging study must be performed.
Further diagnostic tests after the acute phase. Depending on the clinical situation, the following tests can also be performed after the acute phase:
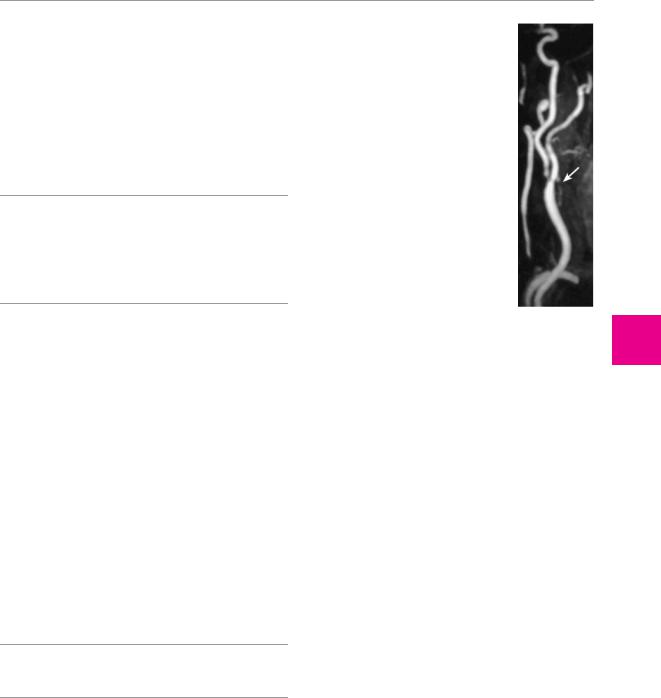
angio-MRI to reveal stenosis of the carotid or vertebral a. (Fig. 6.17);
transthoracic or transesophageal echocardiography to reveal potential sources of emboli in the heart and aortic arch, as well as any dysfunction of the heart valves;
cerebral angiography to reveal stenosis or occlusion of the cerebral blood vessels (also performed in the
acute phase as a part of thrombolytic treatment); andSPECT to demonstrate impaired perfusion (cf.
Fig. 4.13, p. ).
Treatment of Ischemic Stroke. Once the diagnosis of ischemic stroke has been made and an intracerebral hemorrhage has been excluded, the initial goal of treatment is to minimize the amount of brain tissue that will be irreversibly damaged. Brain tissue in the zone of relative ischemia (the ischemic penumbra, p. 99) can be “salvaged” by prompt restoration of its obstructed blood supply.
!Patients with suspected stroke should be immediately transported to an acute care hospital and admitted. Inpatient treatment markedly improves prognosis.
In parallel with the acute measures already discussed, a further treatment strategy should also be settled upon for long-term prevention of recurrent stroke. The appropriate strategy depends on the etiology of the infarct.
General treatment strategies for ischemic stroke are as follows:
keeping the blood pressure relatively high (values up to 200−220 mmHg systolic and 110 mmHg diastolic are tolerable);
stabilization of cardiovascular function (adequate hydration, treatment of heart failure and/or arrhythmia, if present);
blubber blubber
Fig. 6.17 High-grade stenosis of the right common carotid a. in a 72-year-old woman. The MR angiogram, obtained after the injection of contrast medium, reveals high-grade narrowing at the carotid bifurcation (arrow).
treatment of cerebral edema, if present (p. 93); and
in some patients, intravenous thrombolysis within three hours of the onset of symptoms, or intra-arte- rial thrombolysis within six hours; if thrombolysis is contraindicated, aspirin is the drug of choice.
Optimization of oxygen and substrate delivery to the ischemic zone:
monitoring of respiratory function (with blood gas analyses, if necessary, and prophylaxis and treatment of pneumonia);
treatment of pathological metabolic processes that elevate the demand for oxygen and energy (e. g., treatment of fever, suppression of epileptic seizures); and
optimal blood sugar management, with prevention and, if necessary, treatment of hyperor hypoglycemia.
Further therapeutic measures include rehabilitation and prophylactic measures against recurrent stroke:
Early rehabilitation: mobilization (decubitus prophylaxis), physical and occupational therapy, and, if needed, speech therapy.
Prevention of recurrent stroke:
General medical treatment: minimization of vascular risk profile (optimal treatment of hypertension, diabetes mellitus, hypercholesterolemia, or sleep apnea syndrome, if present, and cessation of smoking); treatment of heart failure and/or arrhythmia.
Antithrombotic therapy: the type to be given depends on the etiology of the initial stroke. The following options are available:
inhibition of platelet aggregation (mainly aspirin, but also clopidogrel or aspirin with dipyridamole);
full heparinization and oral anticoagulation
(mainly after cardioor aortoembolic stroke,
Mumenthaler / Mattle, Fundamentals of Neurology © 2006 Thieme All rights reserved. Usage subject to terms and conditions of license.
6
Diseases of the Brain and Meninges
