
Engineering and Manufacturing for Biotechnology - Marcel Hofman & Philippe Thonart
.pdf
J.-F. Cornet, C.G. Dussap and J.-J. Leclercq
This work is supported by the European Space Agency (ESA) through the MELiSSA Project.
References
Acicn Fernandez F. G., Garcia Camacho F., Sanchez Perez J. A., Fernandez Sevilla J.M., Molina Grima E.
(1997). A Model for light distribution and average solar irradiance inside outdoor tubular photobioreactors for the microalgal mass culture. Biotech. Bioeng. 55, 701-714.
Aiba S. (1982). Growth kinetics of photosynthetic microorganisms. Adv. Biochem. Eng. 23, 85-156.
Bohren C.F., Huffman D.R. (1983). Absorption and scattering of light by small particles, John Wiley & Sons Inc.
Brewster M.Q., Tien C.L. (1982). Radiative transfer in packed fluidised beds: dependent versus independent scattering. J. Heal Transfer 104, 573-579.
Brucatto A., Rizzuti L (1997). Simplified modelling of radiant fields in heterogeneous photoreactors. Ind. Eng. Chem. Res. 36, 4748-4755.
Cassano A. E., Martin C. A., Brand! R.J., Alfano O. M. (1995). Photoreactor analysis and design:
Fundamentals and Applications. Ind. Eng. Chem. Res. 34, 2155-2201.
Cornet J.-F.. Dussap C.G, Dubertret G. (1992). A structured model for simulation of cultures of the cyanobacterium Spirulinaplatensis in photobioreactors. Biotech. Bioeng. 40, 817-825.
Cornet J.-F., |
C.G., Gros J.-B. (1994). Conversion of radiant light energy in photobioreactors. |
A.l.Ch.E. Journal. 40, 1055-1066.
Cornet J.-F'., Dussap C.G., Gros J.-B., Binois C., Lasseur C. (1995). A simplified monodimensional approach for modelling coupling between radiant light transfer and growth kinetics in photobioreactors. Chem.
Science. 50, 1489-1500.
Cornet J.-F. (1996). Model parameters for growth of Rhodospirillum rubrum under light limitation in photobioreactors. Technical Note 23.4. ESA contract PRF 141315, M.O.U. ECT/FG/CB/95.205.
Cornet J.-F. (1997). Analysis of a photobioreactor performances for sizing a consumer compartment. ESA report, Biorat Project. Consulting agreement 2011/97.
Cornet J.-F., Marty A., Gros J.-B. (1997). A revised technique for the determination of mean incident light fluxes on photobioreactors. Biotechnol. Prog. 13, 408-415.
Cornet J.-F., Dussap C.G , Gros J.-B. (1998). Kinetics and energetics of photosynthetic microorganisms in photobioreactors. Adv. Biochem. Eng. Biotech. 59, 153-224.
Cornet J -F, Dussap C. G., Leclercq J.-J. (1999). Simulation and model based predictive control of photobioreactors. In: 9tn European Congress in Biotechnology - ECB9, July 1999, Brussels.
Cornet J.-F., Albiol J. (2000). Modelling photoheterotrophic growth kinetics of Rhodospirillum rubrum in rectangular photobioreactors. Biotech. Prog. 16, 199-207.
Dussap C.G. (1988). These de Doctoral es Sciences Physiques, Univ. Blaise Pascal. Serie E, n° 409. Koenigsdorff R., Miller F., Ziegler R. (1991). Calculation of scattering fractions for use in radiative flux
models Int. J Heat Mass Transfer. 34, 2673-2676.
Leclercq J.J. (1998). Technical Note 38.2. ESA contract PO 171686, M.O.U. ECT/FG/MMM/97.012. Mengual X., Albiol J., Godia F. (2000). General Purpose Station 98. ESA Contract, MELiSSA Project;
Technical Note 43.7.
Schuster A. (1905). Radiation through a foggy atmosphere. Astrophys. J. 21, 1-22.
Siegel R., Flowell J.R. (1992) Thermal radiation heal transfer. Hemisphere Publishing Corp. 3rd ed. Spadoni G., Bandini E., Santarelli F. (1978). Scattering effects in photosensitised reactions. Chem. Eng
Science. 33, 517-524.
Van de Hulst H.C. (1981). Light scattering by small particles. Dover publications Inc. 2"d ed.
Wang K.Y., Tien C.L. (1983). Radiative heat transfer through opacified fibres and powders. J. Quant.
Spectrosc Radial. Transfer. 30, 213-223.
238
PARTY
REACTOR ENGINEERING
BIOREACTORS FOR SPACE : BIOTECHNOLOGY OF THE NEXT CENTURY
ISABELLE WALTHER*, BART VAN DER SCHOOT, MARC BOILLAT AND AUGUSTO COGOLI*
*''Space Biology Group ETH-Technopark Technoparkstr. 1 CH-8005 Zurich
Phone: ++41 1 445 12 80 Fax: ++41 1 445 12 71 E-mail walther@spacebiol. ethz. ch
Summary
Space biology is a young and rapidly developing discipline comprising basic research and biotechnology. In the next decades biotechnology in space will play a prominent role in the International Space Station (ISS). Therefore, there is an increasing demand for sophisticated instrumentation to satisfy the requirements of the future projects in space biology. Bioreactors will be needed to supply fresh living material (cells and tissues) either to study still obscure basic biological mechanisms or to develop profitable bioprocesses which will take advantage of the peculiar microgravity conditions. Instruments especially developed for space may be the starting point of new technology uses and lead to interesting spin-offs for Earth-bound research.
1. Introduction
Space biology is a relatively young science that has evolved from scientists' need to better understand the effects of a space environment on living systems. At the beginning of space flight, the main concern was the health of the astronauts; therefore almost all experiments were oriented toward physiology and medicine. Though these two domains are still investigated, the interest in basic research and biotechnology in space has risen drastically in the last years.
On Earth, biotechnology has already been used for centuries to produce or to modify food products such as cheese, wine or beer. But it is in the last 30 years that it has really flourished, thanks the use not only of microorganisms, but also of plants and mammalian cells. With an expanding role in health, environmental protection and agriculture, biotechnology is expected to have a significant impact on our lives in the next decades. One of the key elements for the achievement of biotechnological investigations is the reproducibility of the bioprocess. The usual way is to keep the
241
M. HofmanandP. Thonart (eds.), Engineering and Manufacturing for Biotechnology, 241–251. © 2001 Kluwer Academic Publishers. Printed in the Netherlands.
Isabelle Walther, Bart Van Der School, Marc Boillat and Augusto Cogoli
environmental factors under control by automation and regulation of the cultivation process. For this purpose, the cultivation is performed in a bioreactor that allows the control of the physical parameters of the culture such as temperature, mixing, aeration and pH. The use of a continuous cultivation mode allows avoiding a constant change of the nutritive environment of the cells as in a batch culture.
In space, the use of bioreactors has been very limited to date, but with the upcoming of the international space station, a special attention has evolved for the development of life support systems allowing the recycling of expendable materials (i.e. water, air) and the treatment of waste by-products [1,2,3] as well as for the cultivation of microorganisms, mammalian cells and tissues for food production or medical utilisation.
2. Space bioreactors : instrument
The bioreactors normally used on Earth cannot be used in space for several reasons.
First, most of the materials utilised for the fabrication of a bioreactor are not accepted in space for safety reason. No plastic easily flammable, no large piece of glass, nor any strong acid or base is allowed. Second, the design of the space apparatus is restricted by limitations of size, weight, and power. Third, the absence of sedimentation in space obliges, for example, to fill completely the cultivation chamber of the reactor (zero headspace) to avoid the presence of a gas bubble in the system. In fact, the bubble will not go to the top of the chamber as at Ig but will float somewhere in the middle of the bioreactor creating interference. Finally, as convection movements are also reduced to near zero, nutrient, oxygen and waste products should be efficiently transported by means of medium exchange, perfusion or slow mixing. For these reasons, new types of bioreactors specifically adapted to space investigations had to be developed. Several types of cultivation systems have been designed (Table 1) or are currently under development. The sophistication grade of the devices presented below varies greatly. Some of the instruments consist of simple cultivation chambers with automatic perfusion system for the exchange of medium. Some are much more elaborated and allow automatic regulation of cultivation parameters (pH, oxygen), sampling or fixation of the cells during flight.
242

Bioreactors for space: biotechnology of the next century
2.1. LARGE SPACE BIOREACTORS
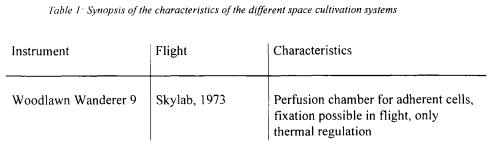
The first bioreactor-like instrument, which has flown in space, was the so-called Woodlawn Wanderer 9 apparatus [4]. It consisted of a fully automated perfusion chamber with devices for light microscopy and a motion picture camera. It was installed aboard the US space station Skylab in 1973. The Space Tissue Loss (STL) system, based on the hollow-fibre technology, provides flexible feeding capabilities, thermal regulation and chemical fixation of the cells. It fits in a mid-deck locker of the Space Shuttle and was used on several flights between 1992 and 1996. To our knowledge only the results of the first flight of the STL-A apparatus with mammalian cells in 1992 have been presented [5]. A set of bioreactors, all based on the principle of the rotating wall vessel (RWV), was developed by NASA at the Johnson Space Centre. Both fed-batch
243
Isabelle Walther, Bart Van Der School, Marc Boillat and Augusto Cogoli
and perfusion systems are available, and oxygenation is achieved by diffusion through a silicone membrane. The first flight of a RWV bioreactor took place with mammalian cells in 1991. This instrument, which provides a very low shear force environment, is now widely used for microgravity simulations on Earth.
Two other devices have been developed recently in Switzerland under ESA (European Space Agency) contract, but have not been flown yet. In the first apparatus, the cell culture is held in a chamber between two plates, each of which carries dividers that interlock to partially divide the chamber. The plates are flat with cylindrical rims. Rotating one of the plates causing the spirals to approach and separate from each other agitates the culture. A portion of at least one plate is porous allowing the oxygen to diffuse into the culture (Patent FR 2724280, 1996). The second apparatus is a bioreactor with a culture volume of 200 ml; it contains an agitator equipped with permeable tubing to provide microgravity-compatible gas exchange together with medium supply and removal. The bioreactor is further equipped with temperature control (Peltier),  control, dissolved oxygen and pressure sensors, sampling ports and optical access ports.
control, dissolved oxygen and pressure sensors, sampling ports and optical access ports.
Its total mass is 30 kg including pumps and electronic box (ESA communication).
2.2. MINIATURE SPACE BIOREACTORS
The instruments described above have the disadvantage of being of a rather large size and certain have no control capacities. Because of their volume they could not be used by. European investigators In fact the two experiment containers used up to now by the European Space Agency (ESA) are of very limited size (65 cc, respectively 385 cc, with dimensions of 80x40x20 mm, respectively 87x63x63 mm).
To palliate this lack, we develop several small culture systems. The first culture chamber was still uncontrolled, but fresh medium was continuously supplied by an osmotic pump [6]. The second instrument was a miniaturised bioreactor named (SBRI) running in a continuous mode, regulated to procure controlled environmental growth conditions and allowing the delivery, on-line, of biological parameters [7,8]. The construction of this bioreactor in such a reduced volume was only possible thanks the use of silicon microtechnology. A short description of these instruments is given in the next chapters.
2.2.7. The DCCS
The Dynamic Cell Culture System (DCCS, Fig. 1) has been developed by the Group of Space Biology at the ETH Zurich in collaboration with Contraves AG. This system is designed for the cultivation of mammalian and plant cells and fits into one Biorack
Type I container (80x40x20 mm). The DCCS consists of a culture chamber of 200 ul supplied continuously with fresh medium by an osmotic pump at a flow rate of  [6]. The DCCS was first tested in a 14-day flight onboard the Soviet biosatellite Biokosmos 9 in 1989. In this mission, the growth and development of plant protoplasts were studied [9], The system worked satisfactorily. The DCCS was tested a second time on the IML-1 mission in 1992. In this investigation, the growth and the effect of microgravity on hamster kidney cells was studied [10]. After 8 days of cultivation, the cell growth was compared between the perfusion and the batch chambers. The cells
[6]. The DCCS was first tested in a 14-day flight onboard the Soviet biosatellite Biokosmos 9 in 1989. In this mission, the growth and development of plant protoplasts were studied [9], The system worked satisfactorily. The DCCS was tested a second time on the IML-1 mission in 1992. In this investigation, the growth and the effect of microgravity on hamster kidney cells was studied [10]. After 8 days of cultivation, the cell growth was compared between the perfusion and the batch chambers. The cells
244

Bioreactors for space: biotechnology of the next century
grew better and they produced more tissue plasminogen activator in the perfusion that in the batch chamber. No effect of the microgravity on the cells per se was observable.
2.1.2. The Swiss space bioreactor: SBR 1
The promising results observed with the DCCS concerning the perfusion chamber drove us to develop a more sophisticated device: a bioreactor for continuous cultivation in space. This controlled miniaturised instrument (Fig. 2) was built in collaboration with the company Mecanex S.A. and the Institute of Microtechnology of the University of
Neuchatel (Switzerland). The SBR I was developed for the cultivation of yeast cells. It fits in one Biorack Type II container (87x63x63 mm) and has a weight of 610 g, medium included [7]. The 3-ml culture chamber is supplied continuously with fresh medium (100 ml) by means of a piezoelectric silicon micropump; a flow and a pressure sensor insure a constant delivery of the fresh medium. The flow rate can be modified among  during cultivation. The pH, temperature and Redox potential values are measured by a microsensor. All the measured data
during cultivation. The pH, temperature and Redox potential values are measured by a microsensor. All the measured data  Redox, flow rate, pressure) are available on-line. The pH is regulated electrochemically to avoid the use of a strong base solution. The culture is agitated by means of a magnetic stirrer if required. Samples were withdrawn with a syringe through a rubber sampling port. This instrument flew aboard the Shuttle missions STS-65 and STS-76, in 1994 and 1996 respectively, to investigate the effect of stirring on the cultivation of yeast cells in space [8]. In fact, the absence of convection in microgravity leads to the formation of gradients in the culture, which might affect the growth of the cells. The gradients cannot form when the culture is stirred. The performances of the SBRI in space flights are presented in the next chapter.
Redox, flow rate, pressure) are available on-line. The pH is regulated electrochemically to avoid the use of a strong base solution. The culture is agitated by means of a magnetic stirrer if required. Samples were withdrawn with a syringe through a rubber sampling port. This instrument flew aboard the Shuttle missions STS-65 and STS-76, in 1994 and 1996 respectively, to investigate the effect of stirring on the cultivation of yeast cells in space [8]. In fact, the absence of convection in microgravity leads to the formation of gradients in the culture, which might affect the growth of the cells. The gradients cannot form when the culture is stirred. The performances of the SBRI in space flights are presented in the next chapter.
245

 Walther, Bart Van Der School, Marc Boillat and Augusto Cogoli
Walther, Bart Van Der School, Marc Boillat and Augusto Cogoli
3. Space bioreactor SBRI: performances in flight
In view of the complexity of the instrument and the limited available space in the experiment container, it is obvious that the use of miniature, microfabricated components offers a distinct advantage. In the following sections, the individual elements will be described in more detail and special attention will be given to the way they are assembled in the bioreactor.
3.1. LIQUID HANDLING
The cell culture has to be continuously supplied with fresh nutrient medium at an adjustable rate. For this, a piezoelectric silicon membrane pump is used. The design of the micropump is based on the original work described in [11]. It consists of a sandwiched glass-silicon-glass structure with the inand outlet in the bottom glass plate. Fluidic connections are made by clamping the pump onto a base plate containing channels, using O-rings to ensure a leak free mounting. The base plate is machined from Vespel® polyimide, a material that has been used for many of the mechanical parts in the bioreactor. Vespel® has an excellent mechanical strength and dimensional stability and can easily be machined. In the first flight of the bioreactor, we noticed that the delivered flow of the pump was highly dependent on the output pressure that has to be supplied.
246

Bioreactors for space: biotechnology of the next century
To overcome this pressure dependence, the improved version of the bioreactor for the second flight was outfitted with a flow sensor. This sensor is used in a closed loop control system that adjusts the driving voltage of the pump to obtain the required output. The flow sensor, based on a modified commercial piezoresistive low-pressure sensor, uses a differential pressure measurement across a flow restriction (Fig. 3).
As a final step in the processing of the sensor wafers, a flow restrictor is etched between two adjacent pressure sensors on the backside. The silicon wafer is subsequently bonded on a glass wafer with through holes for the fluidic connections. The sensor is then diced from the wafer and mounted stress-free on a ceramic substrate, again with through holes for the fluidic connections, using silicone rubber joints. Electrical contacts are made to a metalisation pattern that is screen-printed on the substrate. The mounting of the sensor is thus fully compatible with conventional hybrid electronic assembly techniques that ensure a high reliability at a modest cost. The complete sensor is connected in a fashion similar to the pump, by clamping the ceramic substrate onto a base in which fluidic channels are machined.
Its accuracy is over 2% and it has a full-scale sensitivity of 5 ml h"1. The flow was regular and stable at the five different rates.
3.2. CHEMICAL MEASUREMENT AND CONTROL
To ensure optimal growth conditions, the  of the yeast culture has to be tightly controlled. During normal growth, the organisms produce carbon dioxide and other acidic products which lower the pH of the nutrient medium. In general it is sufficient to neutralise these acids by adding an alkaline solution, and it is not necessary to be able to
of the yeast culture has to be tightly controlled. During normal growth, the organisms produce carbon dioxide and other acidic products which lower the pH of the nutrient medium. In general it is sufficient to neutralise these acids by adding an alkaline solution, and it is not necessary to be able to
compensate |
in the opposite direction. This, however, would require an additional |
dosing system |
as well as special measures to safely contain concentrated alkali. |
Therefore, an alternative, electrolytic method (Fig. 4) has been developed which details have been presented in [12].
247

Isabcllc Walther, Bart Van Der School, Marc Boillat and Augusto Cogoli
The various elements of the control system are assembled so that they are becoming part of the chamber. The sensor chip is mounted on a carrier that is inserted in a hole in the wall of the chamber so that the sensor is virtually part of the wall. This allows direct contact with the culture without creation of dead angles. The sensor is operated in conjunction with a silver / silver-chloride reference electrode connected with the solution through a porous junction.
control system are assembled so that they are becoming part of the chamber. The sensor chip is mounted on a carrier that is inserted in a hole in the wall of the chamber so that the sensor is virtually part of the wall. This allows direct contact with the culture without creation of dead angles. The sensor is operated in conjunction with a silver / silver-chloride reference electrode connected with the solution through a porous junction.
248
