
|
|
|
IV.2.1.2 DOUBLE AND MULTIPLE HECK REACTIONS |
1189 |
||
TABLE 4. (Continued ) |
|
|
|
|
||
|
Arene |
|
|
|
Yield |
Ref- |
|
Alkene |
|
Product |
Conditions |
(%) |
erence |
|
Br |
|
Ph |
|
|
|
Br |
|
|
|
Pd(OAc)2, |
37 |
[18] |
|
Ph |
|
(n-Bu)4NBr, K2CO3, |
|
|
|
Br |
|
|
|
|
||
|
|
LiCl, DMF, 100 °C, |
|
|
||
|
|
Ph |
|
3 d |
|
|
|
Ph |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pd(OAc)2, |
51 |
[46] |
Br |
Br |
Ph |
Ph |
P(o-Tol)3, Et3N, |
|
|
|
|
|
|
MeCN, 100 °C, 3 d |
|
|
|
Br |
|
|
|
|
|
|
Ph |
|
Ph |
|
|
|
Br |
Br |
E |
E |
Pd(OAc)2, |
63 |
[47] |
|
|
|
|
P(o-Tol)3, Et3N, |
E = |
|
|
|
|
|
MeCN, 80 °C, 5 h |
CO2Me |
|
|
Br |
|
|
|
|
|
|
E |
|
E |
|
|
|
aFc = ferrocenyl. |
|
|
|
|
|
|
TABLE 5. Fourfold Heck Reaction on 1,2,4,5- and Related Tetrahaloarenes
Arene |
|
|
|
|
|
|
|
Yield |
|
|||
Alkene |
|
Product |
|
Conditions |
(%) |
Reference |
||||||
|
|
|
|
|
|
|
||||||
X |
X |
R |
R |
|
|
|
||||||
X |
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
R |
R |
|
|
|
|||||
|
|
|
|
|
|
|
|
|||||
|
|
R |
|
X = I, R = CO2Me |
Pd(OAc)2, PPh3, Et3N, |
16 |
[7] |
|||||
|
|
|
|
|
|
|
|
|
|
100 °C, 48 h |
|
|
|
|
|
|
|
X = Br, R = H |
|
Pd(OAc)2, P(o-Tol)3, |
76 |
[45] |
|||
|
|
|
|
|
|
|
|
|
|
Et3N, DMF, 125 °C, 8 d |
|
|
|
|
|
|
|
X = Br, R = Ph |
|
Pd(OAc)2, BnEt3NCl, |
62 |
[8] |
|||
|
|
|
|
|
|
|
|
|
|
K2CO3, LiCl, DMF, |
|
|
|
|
|
|
|
|
|
|
|
|
110 °C, 3 d |
|
|
(Continued )
1190 |
IV |
Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
|
|
||
TABLE 5. (Continued ) |
|
|
|
|
||
Arene |
|
|
|
Yield |
|
|
Alkene |
Product |
|
Conditions |
(%) |
Reference |
|
Br |
|
Ph |
|
|
|
|
|
Br |
|
Ph |
Pd(OAc)2, (n-Bu)4NBr, |
70 |
[18] |
|
|
|
||||
|
Br |
|
|
K2CO3, LiCl, DMF, |
|
|
|
|
|
|
|
|
|
Br |
|
|
Ph |
100 °C, 3 d |
|
|
|
|
|
|
|
|
|
|
Ph |
Ph |
|
|
|
|
|
|
|
|
|
|
|
Br |
Br |
Ph |
Ph |
as above |
45 |
[18] |
Br |
Br |
|
|
|
|
|
|
Ph |
Ph |
Ph |
|
|
|
|
|
|
|
|
|
|
Br |
Br |
Fc |
Fc |
as above, no LiCl, |
17 |
[38] |
|
|
|
|
80 °C, 6 d |
|
|
Br |
Br |
|
|
|
|
|
|
Fca |
Fc |
Fc |
|
|
|
aFc = ferrocenyl. |
|
|
|
|
||
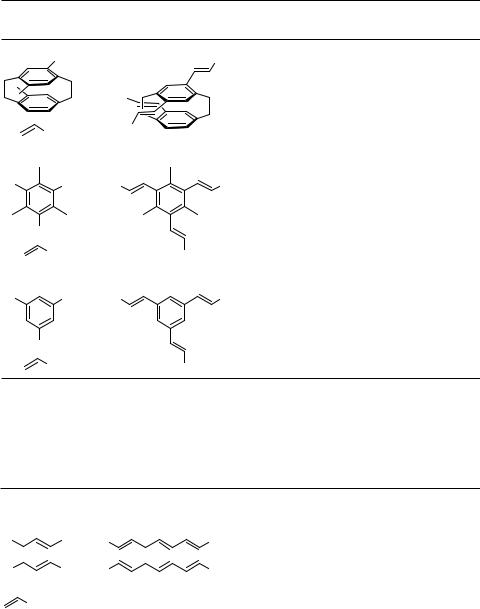
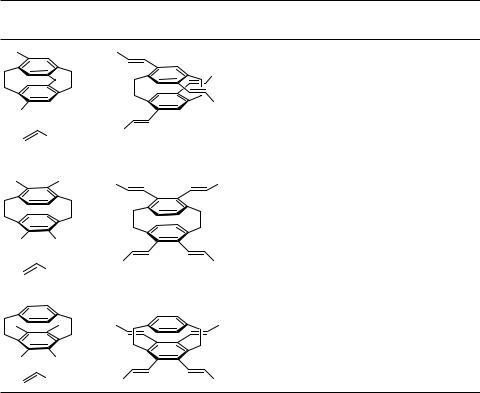
Similarly, the reaction of tetrahaloarenes with alkenes gives rise to the formation of tetraalkenylarenes.
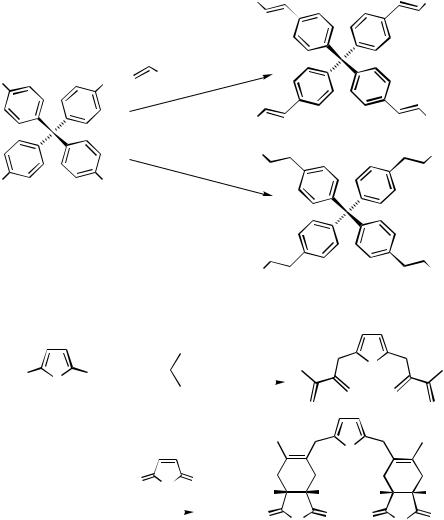
Arenediazonium salts, which are readily available from a large stock of anilines, have proved to be valuable starting materials for Heck reactions since their reactivities exceed even those of the corresponding triflates and hence allow coupling at lower temperatures. Twofold Heck reactions of bisdiazonium salts with various substitution patterns have been investigated (Table 6).[48]
Even a fourfold Heck reaction utilizing the tetradiazonium salt with a tetraphenylmethane framework has been demonstrated with various alkenes to yield the corresponding fourfold coupling products.[49] Allyl alcohols did not couple with the tetradiazonium salt, but the tetrakis( p-iodophenyl)methane did yield the tetraaldehyde upon coupling with allyl alcohol (Scheme 5).[49]
The multiple Heck reaction with oligohaloheteroarenes provided a general access to highly symmetrical, often biologically active, molecules. The failure of 2,6-dihalopyri- dine to undergo a twofold Heck coupling was associated with the presence of a heteroatom.[50] However, 2,5-dihalothiophenes are excellent substrates for a twofold Heck coupling, even with 1,1-dimethylallene, the product of which subsequently undergoes a twofold Diels – Alder reaction (Scheme 6, Table 7).[51],[52]

IV.2.1.2 DOUBLE AND MULTIPLE HECK REACTIONS |
1191 |
TABLE 6. Twofold Heck Reaction of Arenebisdiazonium Salts[48]: E = CO2Me
Arene |
|
|
Yield |
Alkene |
Product |
Conditions |
(%) |
|
|
|
|
+N2 |
|
|
|
|
|
|
|
|
N2+ |
|
|
|
|
|
|
|
E |
|||
|
|
|
|
|
|
|
|
|
2 BF |
– |
|
|
|
|||||||
|
|
|
|
4 |
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO2Me |
|
|
|
|
|
|
|
|
|||||||
|
R1 |
|
|
R1 |
R2 |
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+N2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N2+ |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 BF |
– |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4 |
|
|
 R2
R2
X
+N2 |
2 BF |
– |
N |
+ |
E |
|
|
2 |
|
||
|
4 |
|
|
|
|
|
CO2Me |
|
|
|
|
+N2 |
|
|
|
N2+ |
E |
|
2 BF – |
|
|
||
|
|
|
|
|
|
|
4 |
|
|
|
|
 CO2Me
CO2Me
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
E |
Pd(OAc)2, |
|
83 |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MeOH, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
65 °C, 1 h |
|
|
R1 |
R1 |
|
|
|
||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R2 |
Pd(OAc)2, |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Et OH,80 °C, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 h |
|
|
R1 = H, R2 = CO2Me |
|
|
70 |
|||||||||||||||||||||||||||||
R1 = Me, R2 = CO2Me |
|
|
73 |
|||||||||||||||||||||||||||||
R1 = Me, R2 = Ph |
|
|
|
|
|
|
|
|
|
|
|
|
|
60 |
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
Pd(OAc)2, |
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EtOH, 80 |
°C, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
E |
1 h |
|
|
|
|
|
|
|
|
|
|
|
X = O |
|
|
|
|
|
|
|
|
|
|
|
|
|
80 |
||||||||||
|
|
|
|
|
|
|
|
X = SO2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
60 |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pd(OAc)2, |
60 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EtOH, 80 |
°C, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
E |
1 h |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TABLE 7. Twofold Heck Reaction on Dihaloheteroarenes: E = CO2Et
|
|
|
|
Yield |
Ref- |
Arene |
Alkene |
Conditions |
Product |
(%) |
erence |
|
|
|
|
|
|
I |
I |
Pd(OAc)2, PPh3, |
E |
E |
86 |
[53] |
|
O |
E |
|
|
|
|
|
|
Et3N, DMF, |
|
|
|
|
O
Br |
|
Br |
E Pd(OAc)2, PPh3, |
E |
E |
83–85 |
[51] |
S |
Et3N, MeCN, |
|
S |
|
|
||
|
|
|
|
|
100 °C, 12–20 h
1192 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
R
|
|
|
Pd(OAc)2, EtOH/H2O |
|
||||||
X |
|
X |
|
|
R, 80 °C |
|
|
|
||
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
X = N2+BF4– |
|
|||
|
|
|
|
|
|
|
|
|
R |
|
|
|
|
|
|
|
X = I |
|
OHC |
|
|
|
|
|
|
|
|
|
|
|
||
X |
|
X |
|
|
|
|
|
|
|
|
|
|
|
Pd(OAc)2,Bu4NCl, |
|
||||||
|
|
|
NaHCO3, CH2 |
|
CHCH2OH, |
|
||||
|
|
|
|
|
||||||
|
|
|
|
|
||||||
|
|
|
THF/DMF, r.t., 48 h |
|
||||||
R = Ph (80%); CO2Et (75%), SO2Ph (20%) |
|
|||||||||
|
|
|
|
|
|
|
|
|
OHC |
|
|
|
|
|
|
|
|
Scheme 5 |
|
||
|
|
|
|
|
|
|
Pd(OAc)2, PPh3 |
|
||
|
|
|
|
|
|
|
Ag2CO3, PhMe, |
S |
||
I |
S |
I + |
|
• |
|
|
|
120 °C, 48 h |
||
|
|
|
|
|||||||
|
|
|
||||||||
|
|
|
|
|
|
|
50% |
|
||
|
|
|
|
|
|
|
|
|
||
S
R
R
CHO
CHO
O |
N |
O |
|
|
|
|
|
|
H |
|
H |
H |
|
H |
|
|
Me |
|
|
||||
|
|
|
|
|
|
|
|
|
|
O |
N |
O |
O |
N |
O |
|
|
||||||
|
|
|
|
|
|
||
|
|
|
Me |
|
|
Me |
|
|
|
Scheme 6 |
|
|
|
|
|
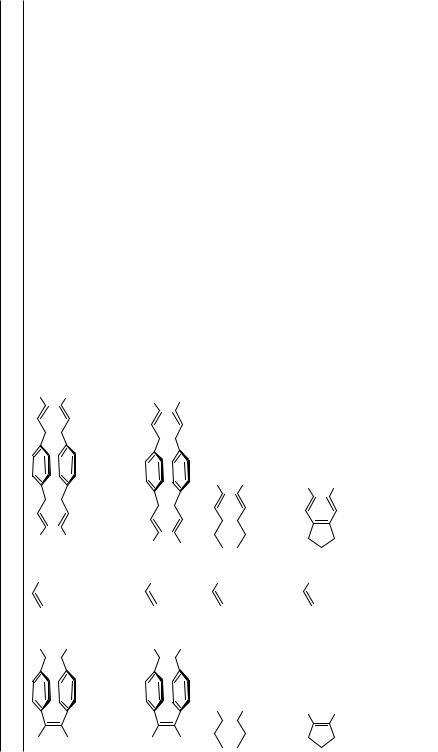
C. MULTIPLE HECK COUPLINGS OF 1,2-DIBROMOCYCLOALKENES AND RELATED COMPOUNDS
Double Heck reactions of vicinal cis-1,2-dihaloalkenes provide an easy access to (E,Z,E)- 1,3,5-hexatrienes, which are valuable building blocks for organic synthesis.[11],[40],[54] The first example of a twofold double, that is, essentially a fourfold, Heck reaction with a bis(dibromo)cycloalkene has been conducted on the strained 1,2,9,10-tetrabromo[2.2] para- cyclophane-1,9-diene, which is available in multigram quantities. The Heck reaction proceeded cleanly under phase transfer conditions, as developed by Jeffery,[41],[42] to give the tetraalkenylated products in moderate to good yields (Table 8). The resulting bishexatrienes
Yield (%) Reference
|
Conditions |
1,2-DibromocycloalkenesandRelatedCompounds |
Oligobromocycloalkenes |
Reactionon |
Alkene |
TABLE8.MultipleHeck |
Oligobromocycloalkenes |
|
|
|
|
|
|
[40] |
[54] |
[40],[54] |
|
[54] |
|
|
|
|
|
|
|
|
|
a |
a |
(20) |
|
(0)38 |
|
|
|
|
|
|
|
|
|
(50)50 |
(80)58 |
|
|
|
|||
|
|
|
|
|
|
|
|
b |
|
a |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3d |
|
C,70° 36h |
C,70° 2d |
|
|
d |
||||
2 |
DMF,100°C, |
|
2 |
DMF,40°C,2 |
|||||||||
NBr, |
|
|
|
|
|
|
NBr, |
|
|
|
|
||
4 |
|
|
|
|
|
|
4 |
|
|
|
|
||
,Bu |
K |
|
above,As |
above,As |
,Bu |
|
K |
||||||
Pd(OAc) |
|
Pd(OAc) |
|
||||||||||
|
|
|
, |
|
|
|
|
, |
|
|
|||
|
|
|
3 |
|
|
|
|
3 |
|
||||
|
|
|
CO |
|
|
|
|
|
CO |
||||
|
|
|
2 |
|
|
|
|
2 |
|
||||
|
|
|
|
|
|
|
Me |
F-4- |
|
|
|
|
=RCHO |
|
|
|
|
|
|
|
CO-4- |
|
|
|
|
||
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
4 |
4 |
|
|
|
|
|
Ar |
|
|
Ar |
Ph |
H |
H |
R |
|
|
|
R |
||
|
|
C |
C |
|
|
|
|||||||
|
|
|
|
|
|
|
6 |
6 |
|
|
|
|
|
|
|
|
|
|
|
Ar= |
Ar= |
Ar= |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ar |
|
|
|
Ar |
R |
|
|
|
R |
Ar |
|
|
|
|
R |
|
|
|
|
Br |
|
|
|
Br |
Br |
|
|
|
Br |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Br |
Br |
Br |
Br |
|
|
|
[11],[56] |
[56] |
[11] |
|
|
|
|
|
|
|
b |
|
|
|
55 |
69 |
57(41) |
|
|
|
N, |
|
|
|
20h |
3 |
|
|
4d |
|||
|
|
Et |
|
h |
||
, |
C,°72 |
90°C, |
90°C, |
|||
|
|
PPh, |
||||
3 |
|
|
|
|
||
2 |
|
DMF,100 |
Asabove, |
Asabove, |
||
|
|
Pd(OAc) |
|
|||
|
|
|
|
Me |
|
t-Bu |
|
|
|
2 |
|
2 |
|
|
|
|
|
R=CO |
R=Ph R=CO |
|
R |
|
R |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
|
|
|
|
||
Br |
|
Br |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N, 3 Et, 3 PPh,
2 Pd(OAc)
R
R
X
[9],[56] |
[9] |
|
|
[56] |
|
|
81 |
82 |
|
|
90 |
|
|
|
N, |
|
N, |
|
|
|
|
3 |
|
|
|
|
|
|
Et |
1d, |
3 |
|
|
|
|
3 |
Et, |
|
|
|
|
h |
, |
|
AgNO 2C,°d, |
|
|
|
Tol)P(-o °C, |
|
|
|
|||
DMF,100°C,40 |
Pd(OAc) |
THF/MeCN,55 kbar10 |
3 |
Arbar5 |
|
|
Pd(OAc) DMSO,20 |
|
|
||||
|
, |
|
, |
|
|
|
|
2 |
|
2 |
|
|
|
Me |
Ph |
|
3 |
|
|
|
CO |
|
SiMe |
|
|
|
|
2 |
|
|
|
|
|
|
R= R= |
|
R= |
|
|
|
|
|
R |
|
|
|
ofyieldIsolated1,2-dicouplingproductafteraromatization. |
ofyieldIsolatedthereducedmonocouplingproduct. |
|
X X=Br |
X=I |
|
|||
|
BrX= |
|
|
|
|
|
|
|
|
|
|
a |
b |
1193

1194 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
upon heating underwent a clean twofold 6 -electrocyclization to yield, after subsequent dehydrogenation, the bisbenzoannelated [2.2]paracyclophanediene derivatives (Scheme 7).
In contrast, the twofold alkenylations of cis-1,2-dibromoethene, 1,2-dibromocyclopen- tene, and 1,2-dibromocyclohexene proceed in better yields under the classical Heck conditions (Table 8).[11] The (E,Z,E)-1,3,5-hexatrienes resulting from 1,2-dibromocycloalkenes undergo 6 -electrocyclizations reasonably cleanly upon heating (130–150 °C) in an inert
Br |
|
Br |
Pd(OAc)2, Bu4NBr |
R |
|
|
|
R |
|||
|
|
|
|
K2CO3 or NaHCO3 |
|
|
|
|
|
||
|
|
|
|
DMF, 40–100 °C, 12 h to 5 d |
|
|
|
|
|
||
Br |
|
Br |
|
|
|
R |
|
|
|
R |
|
|
15–57% |
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|||
+ 4 |
R |
|
|
|
R |
|
|
|
R |
||
|
|
|
|
|
DDQ or S8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
0–80% |
R |
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
||
R = H, SiMe3, CHO, CO2Me, Ph, p-MeO2C-C6H4, p-F-C6H4, p-t-Bu-C6H4, p-Ph-C6H4
Scheme 7
solvent such as di-n-butyl ether or xylene in the absence of oxygen to give the ring-annelated cis-5,6-disubstituted 1,3-cyclohexadienes. In the presence of oxygen these products are easily dehydrogenated to the corresponding ring-annelated benzene derivatives (Scheme 8). Another use of these hexatrienes was demonstrated by the epoxidation of the tetrasubstituted double bond to give dialkenylepoxides, which in turn were selectively ring-opened by Pdcatalyzed reduction. The alcohols resulting from the sixand seven-membered ring epoxides, upon deprotonation at 78 °C, undergo an oxyanion-accelerated Cope rearrangement to give the corresponding trans-cycloalkenones.[55],[56]
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( )n |
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
Br |
|
|
|
|
|
|
|
|
|
|
|
|
[O2] |
|
|||||
|
|
|
|
|
Pd(OAc)2, PPh3, |
|
|
|
R |
|
(n-Bu)2O, N2 |
|
|
|
|
R |
||||||
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
Et3N, DMF, |
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
40−100 °C, |
|
|
|
|
|
150 °C, 5 h |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
( )n Br |
|
|
|
|
|
|
|
( )n |
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
55−81% |
( )n |
|
R |
|
|
50−94% |
|
|
|
R |
|||||||||
n = 1, 2, 3 |
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
1. MCPBA or DMDO |
n = 1, 2, 3 |
|||||||||||||
R = CO2Me, CO2t-Bu, Ph |
79−87% |
|
||||||||||||||||||||
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
2. Pd0, HCO H, 20 °C |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
O |
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
OH |
|
|
|
|
|
|
O |
||||||||
|
|
|
|
|
|
KHMDS, 18-c-6 |
|
|
KHMDS, 18-c-6 |
|
||||||||||||
|
|
|
|
|
|
|
R |
|
|
|
|
|
|
R |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
R |
|
|
THF, –78 °C |
|
|
|
|
|
|
THF, –78 °C |
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
n = 1 |
( )n |
|
R |
|
|
51−94% |
( )n |
|
|
|
R |
||||
|
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
24−83% |
|
|
H |
|
|
|
|
|
|
n = 2, 3 |
|||||
|
|
|
|
|
|
|
|
n = 1, 2, 3 |
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Scheme 8
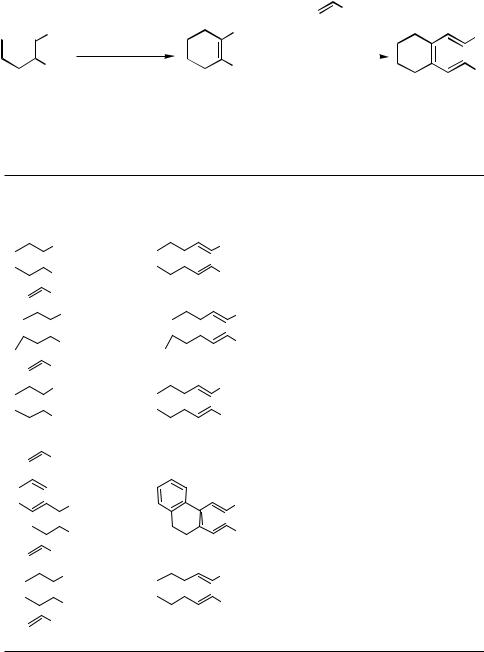
IV.2.1.2 DOUBLE AND MULTIPLE HECK REACTIONS |
1195 |
As alternatives to the 1,2-dihalocycloalkenes, 1-halo-2-perfluoroalkanesulfonyloxycy- cloalkenes may serve as starting materials for twofold Heck reactions. Since they can easily be prepared from the corresponding ketones via the -haloketones and subsequent sulfonylation of the enolates, this sequence provides a straightforward access to various substituted 1,3,5-hexatrienes (Scheme 9, Table 9).[57]

 O 1. LDA, THF, –78 °C, 0.5 h, → 20 °C
O 1. LDA, THF, –78 °C, 0.5 h, → 20 °C
Hal 2. (RFSO2)2O or (RFSO2)2NPh or
RFSO2F
|
R |
|
OSO2RF |
Pd(OAc)2, PPh3, |
|
Et3N, LiCl, DMF, |
||
|
||
|
40 −100 °C |
Hal
RF = CF3 or n-C4F9
Hal = Br, Cl
Scheme 9
R
R
TABLE 9. Twofold Heck Reaction on 1-Halo-2-perfluoroalkanesulfonyloxycyclo-
alkenes[56],[57]
1-Halo-2-perfluoroalkane- |
|
|
|
sulfonyloxycycloalkane |
|
|
Yield |
Alkene |
Product |
Conditions |
(%) |
|
|
|
|
|
|
|
|
|
|
|
|
OTf |
|
|
|
|
|
|
|
E |
|
Pd(OAc)2, PPh3, Et3N, |
|
|||
|
|
|
|
|
|
|
|
Br |
|
|
|
|
|
|
|
E |
|
LiCl, DMF, 90 °C, 96 h |
73 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
E |
E = CO2t-Bu |
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
OTf |
|
|
|
|
|
|
|
|
E |
Pd(OAc)2, PPh3, Et3N, |
86 |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
Br |
|
|
|
|
|
|
|
|
E |
LiCl, DMF, 90 °C, 40 h |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
E |
R = CO2t-Bu |
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
OTf |
|
|
|
|
|
|
|
E |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Br |
|
|
|
|
|
|
|
E |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
OR |
|
|
OR |
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
E |
R = SiMe2t-Bu |
|
Pd(OAc)2, PPh3, Et3N, |
64 |
||||||||||
|
|
|
|
|
|
|
|
|
|
E = CO2t-Bu |
|
DMF, 80 °C, 20 h |
|
|
||||||||
|
|
|
|
|
|
|
|
|
ONf |
|
|
|
|
|
|
|
|
E |
Pd(OAc)2, PPh3, Et3N, |
71 |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
Br |
|
|
|
|
|
|
|
|
E |
LiCl, DMF, 90 °C, 40 h |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
E |
E = CO2Me |
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
ONf |
|
|
|
|
|
|
|
E |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cl |
|
|
|
|
|
|
|
E |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
E |
|
|
|
|
|
|
|
|
|
Pd(OAc) |
, PPh , Et N, |
23 (64)a |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
3 |
3 |
|
|
|
|
|
|
|
|
|
|
|
E = CO2Me |
|
DMF, 75 °C, 8 d |
|
|
||||||||
a Yield of the monocoupling product in parentheses.
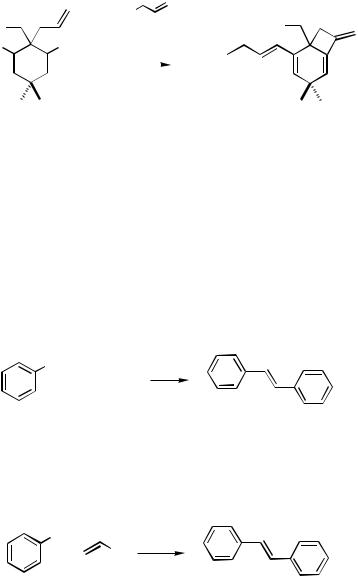
1196 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
D. INTRA–INTERMOLECULAR TWOFOLD HECK COUPLING
OF 1,3-BIS(NONAFLUOROBUTANESULFONYLOXY)CYCLOHEXADIENE
The intra–intermolecular Heck reaction of dienediol bisnonaflates containing an -alkenyl substituent with an external alkene such as an acrylate gives rise to the formation of a bicyclic tetraene by an intramolecular coupling followed by an intermolecular
Heck reaction (Scheme 10).[58]
|
|
|
O |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
t -BuO |
|
|
|
|
|||
Ph |
|
Pd(OAc)2, PPh3 |
|
O |
Ph |
|||||
|
|
|
||||||||
NfO |
ONf |
Et3N, DMF |
|
|
|
|
||||
|
|
|
|
|||||||
|
|
|
80 °C, 12 h |
t-BuO |
|
|||||
|
|
|
|
|||||||
|
|
|
43% |
|
|
|
|
|
||
|
|
|
|
|
|
|
||||
Scheme 10
E. MULTIPLE HECK 1,1- AND 1,2-BISARYLATIONS OF ETHYLENE AND RELATED COMPOUNDS
The twofold Heck arylation of ethylene[13],[59] and ethylene equivalents provides an easy access to stilbene derivatives (Scheme 11, Table 10). In the case of ethylene, the pressure has to be carefully controlled, otherwise styrene derivatives, which are the primary products in this process, will be found as major products. In general, slightly pressurized (1 – 5 bar) reaction conditions are suitable for the twofold coupling and lead to stilbenes in up to 91% yield and with turnover numbers up to 18,200. The linear dependency on ethylene pressure in the arylation of ethylene with aroyl chlorides to give stilbenes (low pressure of ethenyl) or styrenes (high pressure) has been shown previously.[60]
X
,,
+ H2C  CH2
CH2
Pd ,,
Scheme 11
Besides ethylene, ethenylsilanes and ethenyl acetate, which are easy to handle, can yield stilbenes by 1,2-arylation with elimination of the functionality, in good yields (Scheme 12, Table 11).
X
,,
+ Y
Pd ,,
Y = Si(OEt)3, OAc, etc.
Scheme 12

|
IV.2.1.2 |
DOUBLE AND MULTIPLE HECK REACTIONS |
1197 |
|
TABLE 10. Double Heck 1,2-Arylations of Ethylene |
|
|
||
|
|
|
|
|
|
Ethylene |
Product |
Yield (%) |
|
Halorene |
(Pressure) |
(Conditions) |
(Reference) |
|
|
|
|
|
|
Br
Me
Br
X
COCl
R1–3
8 examples
R
R = H or SO3H
20 psi |
Me |
Me
(Pd(OAc)2, P(o-Tol)3, MeCN, 125 °C, 20 h)
120 psi |
X |
X = NHAc
X
(Pd(OAc)2, P(o-Tol)3, DMF, 125 °C, 2 h)
X = CHO
(as above, 8 h, 1 bar ethylene)
1 bar |
R1–3 |
8 examples
R1–3
(Pd(OAc)2, P(o-Tol)3, BnMe2N or N-ethylmorpholine, p-xylene, 120 °C, 4.5–7.5h)
Br 1.5 bar R
R
R = H or SO3H
(Pd(OAc)2, P(o-Tol)3, Et3N, MeCN or NMP, 125 °C)
34 (54)a ([13])
48
([13])
75
([61])
45
([60])
66−72 ([59])
a Yield of the styrene formed is given in parentheses.
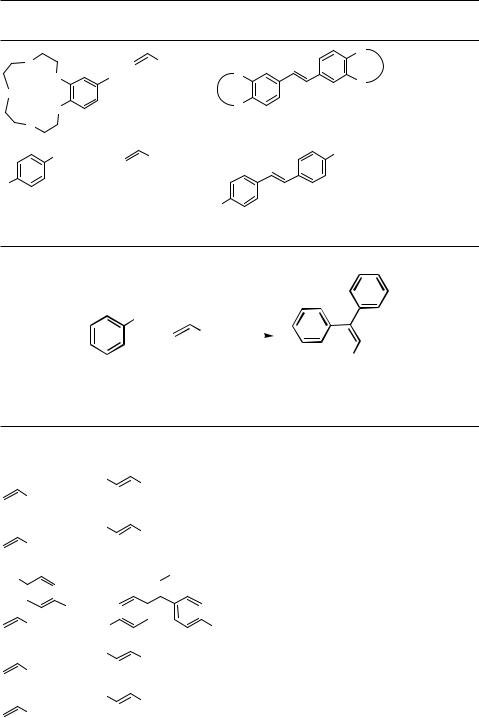
In general, the Heck coupling of aryl halides with terminal alkenes yields styrene derivatives. However, under certain conditions such as with an excess of the aryl halide,[64] at elevated temperatures, under high pressure,[65] and with electron-deficient alkenes, a terminal twofold coupling to yield 1,1-diarylalkene derivatives may take place (Scheme 13,
Table 12).
Under high pressure (10 kbar), the -hydride elimination is retarded and the metastable -alkylpalladium halide may insert into the double bond of a second acrylate
1198 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
|
||
TABLE 11. Double Heck 1,2-Diarylation of Ethylene Equivalents |
|
|||
|
|
Ethylene |
Product |
Yield (%) |
|
Haloarene |
Equivalent |
(Conditions) |
(Reference) |
|
O |
OAc |
O |
|
|
|
|
||
|
O |
I |
O |
|
O |
|
|
O |
|
|
|
|
50 |
|
|
O |
|
O |
|
|
|
|
||
|
O |
|
(polymer supported Pd, Bu3N, MeCN, |
([62]) |
|
|
|
130 °C, 16 h) |
|
|
N2+BF4– |
Si(OEt) |
I |
|
|
|
3 |
|
|
I |
|
|
|
|
|
|
|
I |
62 |
|
|
|
(Pd (OAc)2, PPh3, MeOH, 25 °C, |
([63]) |
|
|
|
30 min) |
|
X
|
,, |
,, |
|
+ |
R |
|
Pd |
|
|
||
|
|
|
|
R
Scheme 13
TABLE 12. Double Heck 1,1-Arylation of Acrylates and Related Compounds
Haloarene |
|
|
Yield (%) |
Alkene |
Product |
Conditions |
(Reference) |
|
|
|
|
PhBr |
Ph |
|
|
|
CO2Me |
Pd(OAc)2, P(o-Tol)3, Et3N, |
78 |
|||||||
|
CO2Me |
|
|
|
|
100 °C, 34 h |
([64]) |
|||||||
|
|
Ph |
||||||||||||
|
|
|
|
|||||||||||
excess |
Ph |
|
|
|
|
|
|
|
|
Pd(OAc)2, P(o-Tol)3, Et3N, |
52 |
|||
PhI |
|
|
|
CO2Me |
||||||||||
|
CO2Me |
|
|
|
|
MeCN, 100 °C, 43 h |
([66]) |
|||||||
|
|
Ph |
||||||||||||
|
|
|
|
|||||||||||
excess |
|
|
|
|
|
|
|
|
CHO |
Pd(OAc)2(PPh3)2, Et3N, |
73 |
|||
HO |
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
I |
|
|
|
|
|
|
|
|
MeCN, 80 °C, 10 h |
([67]) |
|
CHO |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
||||
|
HO |
|
|
|
|
|
|
|
|
OH |
|
|||
PhI |
Ph |
|
|
|
CN |
PdCl2 on modified |
90 |
|||||||
|
CN |
|
|
|
|
montmorillonite, Bu3N, |
([68]) |
|||||||
|
|
|
|
|
|
|
|
|
|
|||||
|
|
Ph |
100 °C, 4 h |
|
||||||||||
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PhI |
Ph |
|
|
|
CO2Et |
Pd(OAc)2(PPh3)2,Et3N, |
76 |
|||||||
|
|
|
|
|||||||||||
|
CO2Et |
|
|
|
|
DMF, 140 °C, 4 h, 10 kbar |
([65]) |
|||||||
|
|
Ph |
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
