

III.3.1 Pd-CATALYZED HYDROGENOLYSIS |
1005 |
D. HYDROGENOLYSIS OF N—N BOND
Azide is one of the easiest functional groups to reduce with heterogeneous Pd catalyst and H2. Thus, even with the less active Lindlar’s catalyst, azides can readily be reduced to amines. Reduction of azido alkynoate ester 84 using Lindlar’s catalyst produced cis- alkenylaminocarboxylic acid ester 85 in 86% yield (Scheme 30).[35]
Tertiary azides can be reduced to primary amines under ambient temperature and low hydrogen pressure (Scheme 31).[36]
Reduction of alkyl azides can also be accomplished under transfer hydrogenation conditions using cyclohexene[37] and formate[38](Scheme 32).
MeO
O
84
X
|
|
|
|
atm H2 |
|
|
|
|
|
|
|
|
|
94 wt % of |
|
|
|||
|
|
|
|
Lindlar,s cat. |
MeO |
H |
|||
|
|
|
|
quinoline |
|
|
|
H |
|
|
|
N3 cyclohexane |
O |
||||||
|
|
|
|
r.t., 3.5 h |
|
|
|
||
|
|
|
|
|
|
|
|
H2N |
|
|
|
|
|
|
|
|
|
85 |
86% |
|
|
Scheme 30 |
|
|
|
|
|||
R |
|
|
3 atm H2 |
|
|
R |
|
||
|
|
|
Pd/C (10%) |
|
|
|
|
||
N3 |
Si |
AcOEt, r.t. |
X |
NH2 |
|
||||
O |
|
|
|
|
O |
|
|||
|
|
|
|
|
|
|
|
||
86 |
|
|
|
|
|
87 |
Si |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X = O, |
R = H |
90% |
|
||
|
|
|
|
X = S, |
R = H |
90% |
|
||
|
|
|
|
X = O, |
R = Me |
75% |
|
||
Scheme 31
O |
|
|
O |
|
N3 |
Pd/C |
|
NH2 |
|
O |
|
O |
||
cyclohexene |
|
|||
|
|
|
|
|
88 Ph |
NH4+ HCO2 |
89 82% Ph |
||
|
|
|
|
|
OH |
176 wt % of |
|
OH |
|
Pd/C |
|
|||
N3 |
MeOH |
|
NH2 |
|
60 °C, 0.1 h |
|
|||
N3 |
|
H2N |
|
|
|
|
|||
OH |
|
|
OH |
|
90 |
|
|
91 |
71% |
Scheme 32

1006 III Pd-CATALYZED CROSS-COUPLING
Functional groups that are stable to reduction during azide hydrogenolysis include benzyl ester, alkyl chloride, benzyl ether, epoxide, alkoxy imine, hydrazone, nitrile, aldehyde, and ketone (Scheme 33).[39]–[43]
The above list of functional groups can be made to react along with the azido group under more vigorous conditions or extended reaction time. For example, the olefin and benzylic amine groups were further hydrogenolyzed under these conditions (Scheme 34).[44],[45]
|
|
|
|
|
|
|
|
|
1 atm H2 |
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
7 wt % of |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
O |
|
N3 |
Lindlar,s catalyst |
|
|
|
|
|
O HN |
O |
|||||||||||||||
|
|
|
|
|
|
|
|
OMe |
MeOH |
|
|
|
|
|
|
|
|
|
|
|
OMe |
|||||||
Ph |
O |
NH |
|
r.t., 5 h |
|
|
Ph |
O |
|
|
NH |
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
92 |
|
|
+ |
|
O |
|
|
|
|
|
|
|
93 |
|
56% O |
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
O |
O O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
PhCH2O |
|
|
|
|
|
1 atm H2 |
|
|
PhCH2O |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
PhCH2O |
|
|
|
|
|
|
|
|
17 wt % of |
|
PhCH O |
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
Pd/C(10%) |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
Cl |
2 |
|
|
|
|
|
|
|
|
Cl |
|
|
||||||||
|
|
|
|
|
|
|
|
EtOH, AcOEt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|||||||||
|
|
|
|
|
r.t., 36 h |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
PhCH2O |
|
|
|
H |
|
PhCH2O |
|
|
H |
|
|
|
|
|
||||||||||||||
|
N3 |
|
|
|
|
|
|
|
NH2 |
|
|
|
|
|
||||||||||||||
94 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
OH |
|
OMe |
|
|
atm H2 |
|
|
95 |
|
|
|
|
OMe |
|||||||||||||
HO |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
N |
|
|
3 wt % of |
|
|
|
|
|
|
N |
||||||||||||||||||
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
HO |
|
|
|
|
|
|
|
|
|
N3 |
|
|
Pd/CaCO3 |
|
|
|
|
|
|
|
|
|
NH2 |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EtOH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
r.t.,12 h |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
HO |
|
|
|
|
|
97 |
|
|
|
|
|
|
|
|
98 |
|
96% |
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
96 |
77% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
(CH2)8
 (CH2)6
(CH2)6
N3 |
|
|
|
|
N |
|
N |
|
|
|
|
||||
99 |
|
|
|
|
MeO |
||
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
CN |
|
|
N |
|
|
|
N |
||
|
|
|
|
N3 |
|||
|
|
|
|
|
|||
O |
|
N |
|
N |
|||
|
|
|
|
|
|||
|
|
|
|
101 |
|
|
|
|
|
|
|
|
|
||
1 atm H2
16 wt % of Lindlar,s catalyst
EtOH H2N r.t., 1.5 h
3.4 atm H2
55 wt % of Lindlar,s catalyst dioxane/EtOH r.t.,18 h
Scheme 33
H |
|
|
|
H |
|
|
|
|
(CH2)8 |
|
|
|
|
|
|
||
|
|
(CH2)6 |
||||||
|
|
|
|
|
|
N |
|
N |
|
|
|
|
|
|
|
||
100 |
80% |
MeO |
||||||
|
|
|
||||||
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
CN |
||
N |
|
|
|
NH2 |
||||
|
|
|
|
|||||
|
|
|
|
|
|
|||
O N |
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
102 |
84% |
|
|
|||
|
|
|
|
|||||

|
|
|
|
|
III.3.1 Pd-CATALYZED HYDROGENOLYSIS 1007 |
||||||||
|
|
|
|
|
2.8 atm H2 |
|
|
|
|
|
|
|
|
|
|
|
|
CO2Et |
24 wt % of |
|
|
|
|
|
|
CO2Et |
|
O |
|
Pd/C(10%) |
O |
|
|
|
|||||||
|
|
MeOH |
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
P |
|
N3 |
r.t., 1 h |
|
P |
|
|
|
NH2 |
|||
t-BuO |
|
|
|
|
|
|
t-BuO |
|
|
|
|
|
|
OBu-t |
103 |
|
|
OBu-t |
|
||||||||
|
|
|
|
|
|
|
|
|
|
104 |
89% |
||
|
|
N3 |
Ph |
atm H2 |
H2N |
Ph |
|
||||||
|
|
|
|
|
20 wt % of |
|
|
|
|
|
|
|
|
|
|
|
|
N |
Pd/C(10%) |
|
|
|
N |
H |
|
||
|
|
|
|
MeOH |
|
|
|
|
|
|
|||
|
|
|
|
OH |
r.t., 10 h |
|
|
|
|
|
OH |
||
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
105 |
|
|
|
|
|
106 |
95% |
|
|
|
|
|
|
|
Scheme 34 |
|
|
|
|
|
|
|
|
The azide functional group is therefore a convenient precursor to primary amines. The amine formed may further react with other functional groups under the reaction conditions. Thus, the reduction of azido diester 107 provided pyrrolidone 108 after cyclization to the lactam (Scheme 35).[46]
In the presence of formaldehyde the initially formed primary amine further underwent a double reductive amination reaction to give the N,N-dimethylamine product (Scheme 36).[47]
Other examples of intramolecular reductive amination reactions resulting from amines formed from azido compounds are shown below. Excellent diastereoselectivities were observed for the reduction of the intermediate imines in the latter two cases (Scheme 37).[48]–[50]
Intramolecular alkylation of the amine formed can also occur to give cyclic compounds. Electrophiles include mesylate,[51] tosylate,[52] epoxide,[53] alkyl bromide,[54] sulfate,[55] and imino ether[56] (Scheme 38).
Other N—N bonds such as those in hydrazone 129,[57] azine 131,[58] and tetrazolopyrimidinone 133[59] can readily be hydrogenolyzed (Scheme 39).
|
|
|
|
|
2.1 atm H2 |
|
|
|
||
|
CO |
Et |
|
|
9 wt % of |
|
|
CO2Et |
||
|
|
|
Pd/C(10%) |
|
|
|||||
|
2 |
|
|
|
|
|
|
|||
|
|
|
|
|
EtOH |
|
|
|
||
N3 |
CO2Et |
|
r.t., 2 h |
|
|
O |
||||
|
|
|
|
|
N |
|||||
|
107 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H 108 81% |
||
|
|
|
|
|
|
|
|
|
||
|
|
|
|
Scheme 35 |
|
|
|
|||
O |
|
|
H2, Pd/C(10%) |
|
|
O |
||||
N3 |
OMe |
MeOH |
Me2N |
|
OMe |
|||||
formaldehyde |
|
|||||||||
|
|
|
|
|
|
|||||
HO 109 |
|
|
|
|
|
|
|
|
HO 110 |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
Scheme 36 |
|
|
|
|||

1008 III Pd-CATALYZED CROSS-COUPLING
F
N3 |
|
|
O |
OH |
H2, Pd/C(5%) |
|
|
|
|
|
MeOH, H2O |
||
|
|
|
H |
|
||
|
|
|
|
|
|
|
|
|
|
HO |
OH |
|
|
|
111 |
|
|
|
||
|
|
O |
|
atm H2 |
||
|
|
|
|
|
Pd/C(10%) |
|
|
|
|
|
N3 |
MeOH |
|
O |
|
|
O |
|
r.t., 3 h |
|
|
|
|
|
|
||
113 |
|
|
|
|||
|
|
|
N3 |
|
atm H2 |
|
|
|
|
H |
|
5 wt % of |
|
O |
|
|||||
|
Pd/C(10%) |
|||||
|
|
|
|
O |
||
HO |
|
|
|
AcOEt |
||
|
|
|
|
|||
|
|
|
O |
r.t., 12 h |
||
|
|
|
|
|||
O |
|
|
O |
|
|
|
115
Scheme 37
F
 NH
NH
HO
OH OH
112 100%
HN
O  O
O
114 65%
94% de
O 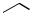 NH
NH
H
O
O
HO
O
116 92%
O O |
|
|
H |
O |
|
MsO |
O |
|
MsO |
||
|
||
117 |
N3 |
MeOCO O
O O
O
TBDMS
1.atm H2
6 wt % of Pd black EtOH, 15 h
2.AcONa, EtOH
1.atm H2
9 wt % of Pd/C(10%)
EtOAc r.t., 3 h
N3 OTs
119
O N3
N3
 O
O
O
O
Ph
Si
Ph Bu-t
121
2.Et3N
DMF 100 °C
atm H2
5 wt % of Pd black
EtOH r.t., 24 h
O
TBDMS
Scheme 38
 O
O
O
H

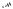 O
O
N
 O
O
118 81%
O MeOCO O
TBDMS
H H
H
NH
120 70% as the N-acyl derivative
OH OH
H H
NH 

 OH
OH
OH
OH
122 75%

|
|
|
|
|
|
|
|
|
III.3.1 |
Pd-CATALYZED HYDROGENOLYSIS 1009 |
|||
AcO |
|
|
|
|
|
OMe |
1. atm H2 |
HO |
OAc |
||||
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
Pd/C(10%) |
|
|
|
|
HO |
N3 |
|
|
|
|
|
|
|
r.t., 2 h |
N |
|
||
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
2. NaOAc |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
MeOH |
H |
|
||
Br |
|
|
|
|
|
|
|
|
reflux |
|
|
|
|
123 |
|
|
|
|
|
|
|
|
|
|
124 |
58% over two steps |
|
|
|
|
|
O SO2 |
2 atm H2 |
|
|
OMe |
|||||
|
|
|
|
|
H |
|
|||||||
|
|
|
|
|
|
O |
Pd/C |
|
|
||||
|
|
|
|
|
|
THF, H2O |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
OSO3− |
|||
|
|
|
|
|
|
|
|
|
r.t., 2 h |
|
NH |
||
|
|
125 N3 |
|
|
126 |
78% |
O |
||||||
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
2 atm H2 |
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
7 wt % of |
|
HN |
N |
|
N3 |
|
H |
|
|
|
|
|
Pd black |
|
||||
|
|
|
|
|
|
|
|
H |
|||||
|
O |
|
|
|
|
|
EtOH |
|
|
||||
|
N |
|
|
|
|
|
|
|
|||||
|
|
|
|
|
O |
r.t., 18 h |
|
|
O |
||||
H |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
N |
|
|
|
H |
OH |
||||
|
|
O |
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
MsO |
|||
MsO |
H 127 |
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
128 94% |
||||
Scheme 38 (Continued)
HN
|
|
N |
|
|
|
|
|
|
6 atm H2 |
|
|
NH2 |
||||||||
|
|
|
|
|
|
OMe |
Pd/C(5%) |
|
|
|
|
|
|
OMe |
||||||
|
|
|
|
|
|
r.t., 4 h |
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
129 |
O |
|
|
|
|
|
|
130 |
|
O |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
95% |
|||||||
|
|
N |
|
|
OMe |
1 atm H2 |
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
Pd(OH)2/C(20%) |
|
|
|
|
|
|
|
|||||||||
|
|
NH |
|
|
|
|
|
|
|
|
|
|
|
|
NH2 |
|||||
|
|
|
|
|
|
|
|
DME/H2O |
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
HCl, r.t. |
|
|
|
|
|
|
|
|||
THPO |
131 |
|
|
|
|
|
|
|
|
|
|
|
THPO |
132 |
89% |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
N |
N |
2 atm H2 |
|
|
NH2 |
|
|
|
||||||||||
|
|
N |
|
|
|
|
|
|
22 wt % of |
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
Pd/C(10%) |
|
N |
|
|
|
|
|
|||||
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
MeCN |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
O |
|
|
|
|
|
|
r.t., 50 h |
O N |
|
|
|
|
||||||
|
|
N |
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
133 |
|
|
|
|
|
|
|
134 |
87% |
|||||||
Scheme 39

1010 |
III Pd-CATALYZED CROSS-COUPLING |
E. HYDROGENOLYSIS OF EPOXIDE
Regioselectivity in the epoxide ring opening is also controlled by similar factors affecting the opening of cyclopropanes, with aryl substituent providing the greatest influence. When an aryl substituent is on the epoxide ring, the benzylic C—O bond is always hydrogenolyzed (Scheme 40).[60]
Since azide is reduced so easily, it is reduced first under the reaction conditions before the epoxide ring hydrogenolysis. Because the epoxide ring opening is quite regioselective, the initially formed amine must not have influenced the regioselectivity of the epoxide ring opening. Similarly, the presence of the hydroxymethyl substituent and steric hindrance around the benzylic position in 137 were not able to prevent the benzylic C—O bond cleavage (Scheme 41).[61] The hydrogenolysis proceeded with inversion of configuration, which is the predominant occurrence with Pd catalysts.
The ester carbonyl also did not exert any influence on the epoxide opening of 139 (Scheme 42).[62] Steric control leads to the exclusive formation of the tertiary alcohol 140.
Under the hydrogenolysis conditions used for the opening of epoxide, other benzylic C—O bonds may also react. For example, the benzylic C—O bond of the acetonide 141 was cleaved to an extent of 80% (Scheme 43).[63]
N3
O
135
H2
15 wt % of Pd/C(10%) EtOH, CHCl3 r.t., 6 h
Scheme 40
NH2
OH
136 69%
atm H2
133 wt % of Pd/C(10%)
TMS |
O |
EtOH, 1M NaOH |
TMS |
|
|
|
|
OH |
||||||
|
|
OH |
−60 °C, 6 h |
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
OH |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
137 |
|
|
|
|
|
|
|
138 |
97% |
|||
|
|
|
|
|
|
Scheme 41 |
|
|
|
|
|
|
||
|
|
|
|
|
|
6.1 atm H2 |
|
|
|
|
|
|
||
|
|
|
|
|
|
15 wt % of |
|
|
|
|
|
|
||
|
OMe |
O |
Pd/C(10%) |
OMe |
|
O |
|
|||||||
|
|
|
|
|
|
EtOH |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|||
O |
|
|
OMe |
|
r.t., 15 h |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
O |
|
|
|
|
|
OMe |
|||
|
|
|
|
|
|
|
|
|
|
|||||
|
|
O |
|
|
|
OH |
|
|||||||
139 |
|
|
|
|
|
|
|
140 |
95% |
|
|
|
||
|
|
|
|
|
|
Scheme 42 |
|
|
|
|
|
|
||

|
|
|
|
III.3.1 Pd-CATALYZED HYDROGENOLYSIS 1011 |
||||
|
|
atm H2 |
|
|
|
|
|
|
|
O |
100 wt % of |
|
O |
|
|
|
|
|
O |
Pd/C(5%) |
|
O |
|
|
OH |
|
|
EtOH, AcOH |
|
|
|
||||
|
H |
r.t. |
|
OH |
+ |
|
OH |
|
|
|
|
||||||
|
|
|
|
|
|
|||
|
|
|
|
|
|
|||
|
O |
|
|
|
|
|
||
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
141 |
|
|
|
142 |
12% |
143 |
80% |
|
Scheme 43
Further reduction completed the hydrogenolysis to the diol. In this case a lower catalyst charge or the use of a less reactive Pd catalyst, such as Pd/CaCO3 or Pd/BaSO4, and a neutral solvent may help to control the overreduction. Transfer hydrogenation conditions using 1,4-cyclohexadiene as the hydrogen source gave 142 in 76% yield along with 16% of the deprotected triol.
Effective reaction conditions to prevent the benzylcarbamate functional group from hydrogenolysis is probably impossible during the epoxide ring opening of 144 due to the higher reactivity of the Cbz group (Scheme 44).[64] The corresponding exo-epoxide was more reactive toward hydrogenolysis and gave the aminol in 86% yield.
Besides aryl-directed ring opening, regioselective opening via allylic activation has also been reported for 146 (Scheme 45).[65] The olefin was not reduced under the reaction conditions.
The use of Pd/CaCO3 provided diol 149 from 148 in high yield without the loss of the benzylic hydroxy group (Scheme 46).[66] Hydrogenolysis of the benzylic hydroxy group, if necessary, can be accomplished with Pd/C at 75 °C.
Mesylate and tosylate functional groups are usually not cleaved under Pd-catalyzed hydrogenolysis conditions. Thus, it was possible to prepare hydroxy tosylate 152 from 151 in good yield (Scheme 47).
Pd-catalyzed transfer hydrogenolysis conditions have also been applied to epoxide opening. Hydrogenolysis of representative examples with ammonium formate are presented in Scheme 48.[67]
|
|
|
|
|
CO2CH2Ph |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
NH |
|
||
|
|
|
H |
N |
H |
|
|
H2, Pd/C |
|
H |
|||||||
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
H |
|
|
|
|||||||
H |
|
|
|
|
|
MeOH |
|
|
|
||||||||
|
|
|
O |
H |
|
|
|
|
|
|
HO |
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
144 |
|
|
|
|
|
|
|
145 |
76% |
|
|||
|
|
|
|
|
|
|
Scheme 44 |
|
|
|
|
|
|
||||
O |
|
|
|
|
|
|
|
|
O |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
H2, Pd/C |
|
|
|
|
|
|
||
O |
O |
|
|
O |
|
|
OH |
||||||||||
|
|
|
OH |
|
|
AcOEt |
|
|
|
|
|
OH |
|||||
O |
|
|
|
|
|
|
|
O |
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||
146 |
|
|
|
|
|
|
|
|
|
147 |
|
|
|
||||
Scheme 45

1012 III Pd-CATALYZED CROSS-COUPLING |
|
|
|
|
|
|
|
|||||||||||||||||||
|
|
O |
|
|
|
H2 |
|
|
|
|
OH |
|
H2 |
|
|
OH |
||||||||||
|
|
|
|
|
|
|
15 wt % of |
|
|
|
|
|
|
|
|
|
|
|
10 wt % of |
|
|
|
||||
|
|
|
|
|
|
|
Pd/CaCO3(5%) |
|
|
|
|
|
|
|
|
|
|
|
Pd/C(10%) |
|
|
|
||||
|
|
|
|
|
|
|
r.t., 115 h |
|
|
|
|
|
|
|
|
|
|
|
EtOH |
|
|
|
||||
|
|
OH |
|
|
|
|
|
|
|
|
|
|
|
OH |
|
75 °C, 92 h |
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
CF3 |
|||||||||||||
CF3 |
|
|
|
|
CF3 |
|
|
|
|
|||||||||||||||||
|
148 91% ee |
|
|
|
|
|
|
149 92% |
|
|
|
|
|
|
|
|
|
150 95% ee |
||||||||
|
|
|
|
|
|
|
|
|
|
|
Scheme 46 |
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
H2 |
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
10 wt % of |
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
Pd/C(5%) |
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
EtOH |
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
O OTs |
|
r.t., 24 h |
|
|
|
OH OTs |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CF3 |
151 |
|
|
|
|
|
|
|
|
|
|
CF3 |
152 84% |
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
Scheme 47 |
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
NH4HCO2 |
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
8 wt % of |
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
O |
Pd/C(5%) |
|
|
OH |
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
MeOH |
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
reflux, 2 h |
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
153 |
|
|
154 |
100% |
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
OH |
|
|
|
|||||
|
|
|
|
|
|
|
CO2Me |
|
|
|
|
|
Ph |
|
|
CO2Me |
|
|
|
|||||||
|
|
|
|
Ph |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
155 |
|
|
156 |
95% |
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
OH |
|
|
|
|||||
|
|
|
|
|
|
|
CO2Me |
|
|
|
|
|
Ph |
|
|
CO2Me |
|
|
|
|||||||
|
|
|
|
Ph |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
157 |
|
|
158 |
95% |
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
Ph |
|
|
|
|
|
Ph |
|
|
|
|
|
|
|
|||||||||
|
|
|
|
159 |
|
|
160 |
70% |
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
|
|
|
|
|
|
|
161 |
|
|
162 |
55% |
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
Scheme 48 |
|
|
|
|
|
|
|
||||||||
F. HYDROGENOLYSIS OF AZIRIDINE
Again, aryl substituent has the greatest directing effect on the regioselectivity of aziridine ring opening. Both inversion and retention of configurations at the carbon center have been observed. Extensive loss of chirality was observed during the cleavage of the C—N

III.3.1 Pd-CATALYZED HYDROGENOLYSIS |
1013 |
bond in methyl ester 163 (Scheme 49).[68] Better stereoselectivity was observed when the t-Bu ester was used.
The stereochemistry of hydrogenolysis of 2-methyl-2-phenylaziridine with Pd(OH)2/C catalyst can favor either retention or inversion of configuration depending on the solvent used.[69] Under neutral reaction conditions, retention of configuration (68%) was observed in benzene whereas inversion of configuration occurred to an extent of 88% in EtOH. With N-acyl-2-methyl-2-phenylaziridine, both benzene and EtOH solvents gave inversion of configuration as the major reaction pathway in 93% and 88% yields, respectively. The stereoselectivity observed in EtOH could be completely reversed with the addition of NaOH and KI. Under these basic conditions, 100% retention was observed with 2- methyl-2-phenylaziridine. Although this reversal was not observed with N-acyl-2-methyl- 2-phenylaziridine, under the basic conditions but in the absence of KI the stereochemistry of hydrogenolysis did improve to 97%. These results were explained based on the affinity of Pd for the neutral nitrogen (in neutral pH) and anionic nitrogen (in basic conditions). With the Pd preferably complexed with the anionic nitrogen because of the nitrogen’s increased electron-donating character and also with the phenyl ring, retention of configuration was observed. Since Pd has a low affinity for the neutral nitrogen, complexation with the Pd occurs primarily with the phenyl ring only and from the backside of the C—N bond, resulting in the observed inversion of configuration.
Neither the amide carbonyl in 165[70] nor the hydroxymethyl group in 167[71] influenced the out come of the hydrogenolysis (Scheme 50).
atm H2 Pd(OH)2/C
CH2Cl2
|
|
|
|
N |
CO2R |
|
|
|
|
|
|
CO2R |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H2N |
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
||
163 |
|
|
|
|
|
|
|
164 |
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
R = Me |
88% yield, 72% de |
||||
|
|
|
|
|
|
|
|
|
|
|
R = Bu-t |
84% yield, 92% de |
||||
|
|
|
|
|
|
|
|
|
Scheme 49 |
|
|
|
|
|
||
|
|
|
|
H |
|
54 atm H2 |
|
|
|
|
|
|
|
|||
|
|
|
N |
|
13 wt % of |
|
|
|
|
|
|
|
||||
|
|
|
|
Pd/C(5%) |
|
|
|
|
|
CONH2 |
|
|||||
|
|
|
|
CONH2 MeOH |
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
60 °C, 14 h |
|
|
|
|
|
NH2 |
|
||
165 |
|
|
|
|
|
|
|
|
|
166 |
90% |
|
||||
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
at 90% conversion |
|
||||
|
SO2Ph |
atm H2 |
|
|
|
|
|
|
SO2Ph |
|||||||
|
7 wt % of |
|
HN SO2Ph |
|||||||||||||
|
|
|
|
|
|
Pd/C(10%) |
|
HN |
|
|||||||
|
N |
|
|
|||||||||||||
|
95% EtOH |
|
|
|
OH |
|
+ |
OH |
||||||||
|
|
|
|
OH |
r.t., 1 h |
|
|
|
|
|||||||
|
|
|
|
Ph |
|
|
|
Ph |
||||||||
Ph |
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||
167 |
|
|
|
|
|
|
|
168 |
99% |
|
|
|
169 |
1% |
||
|
|
|
|
|
|
|
|
|
Scheme 50 |
|
|
|
|
|
||

1014 |
III Pd-CATALYZED CROSS-COUPLING |
In the latter case, only 1% of benzylic sulfonamide 169 was observed resulting from the cleavage of the nonbenzylic C—N bond. A similar substrate 170 was hydrogenolyzed in 99% yield using transfer hydrogenation conditions with formic acid as the hydrogen source (Scheme 51).[72],[73]
In the absence of benzylic electronic effect, the ester group on aziridines 172[74] and 174[75] directed the C—N bond cleavage effectively to give -amino esters (Scheme 52).
For comparison, the hydrogenolysis of the hydroxymethyl derivative proceeded at the less hindered C—N bond (Scheme 53).
For 172, the removal of the N-benzyl group occurred within the first 30 min of the reaction followed by the hydrogenolysis of the aziridine ring, whereas the N-methyl- benzyl protecting group remained attached throughout the reaction for 174. Removal of the N-methylbenzyl group can be accomplished at a higher temperature and in an acidic solvent as reported for the hydrogenolysis of 178 (Scheme 54).[76]
SO2Tolyl |
HCO2H |
|
|
|
||||||||
|
|
|
|
|
Pd black |
HN |
SO2Tolyl |
|||||
N |
|
|
EtOH, 70 °C |
|
||||||||
Ph |
CO2Me |
|
|
|
|
|
|
Ph |
|
CO2Me |
||
170 |
|
|
|
|
|
|
|
171 |
89% |
|||
|
|
|
|
|
Scheme 51 |
|
|
|
||||
O |
|
|
1 atm H2 |
O |
|
|
||||||
|
|
O |
|
|
10 wt % of |
|
O |
|
||||
|
|
|
|
Pd/C(10%) |
|
|
||||||
|
|
|
|
|
EtOH |
|
|
|
||||
|
|
|
|
|
r.t., 3 days |
H2N |
|
|
||||
H N |
CO2Et |
|
|
|
|
|
CO2Et |
|||||
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
||
172 |
CH2Ph |
|
|
|
|
|
173 |
|||||
|
|
|
|
|
100% |
|||||||
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
H2 |
|
|
|
||||
|
|
|
|
|
20 wt % of |
Ph |
|
|
||||
|
|
|
Ph |
Pd(OH)2/C |
|
|
||||||
|
|
|
|
|
AcOH |
|
|
|
||||
|
N |
CO2Et |
r.t., 3 h |
HN |
|
|
||||||
|
|
|
|
|
|
|
CO2Et |
|||||
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
174 H |
|
|
|
|
|
175 80% |
||||||
|
|
|
|
|
Scheme 52 |
|
|
|
||||
|
|
|
Ph |
|
H2 |
|
|
|
||||
|
|
|
|
20 wt % of |
|
|
|
|||||
|
|
|
|
|
|
Pd(OH)2/C |
|
|
|
|||
|
|
N |
CH2OH |
|
AcOH |
HN |
Ph |
|||||
|
|
|
|
|
|
r.t., 3 h |
|
OH |
||||
|
|
|
H |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
176 |
|
|
|
|
|
|
177 95% |
||
Scheme 53
