
- •Types of Organic Reactions
- •Isomerism
- •Types of Organic Reactions
- •1.1 Classification of organic reactions by type of chemical bonds braking
- •Classification of organic reactions by nature of reagent.
- •Classification by the type of organic reactions
- •Classification by number of molecules, which take part in the slowest step of organic reaction (by order reaction)
- •Isomerism
- •2.1. Structural Isomerism
- •Stereoisomerism
Classification of organic reactions by nature of reagent.
Electrophiles
electron-deficient species that tend to accept electron(s)
possess an empty orbital to receive the electron pair
cations or free radicals seeking electron-rich centres
Nucleophiles
electron-rich species that tend to seek an electron-deficient site for reaction
possess lone pairs of electrons
anions or molecules with lone pairs of electrons
Nature |
Electrophile |
Nucleophile |
||
Cation |
Free radical |
Anion |
Molecule with lone pair of electrons |
|
Example |
Br+, Cl+, NO2+, R+, RCO+, SO3H+ |
H•, Br•, Cl•, I•, R•, HO•, CH2=CHCH2•, |
Cl–, Br–, I–, RO–, CN–, OH–, RCOO– |
H2O, ROH, ROR, NH3, RNH2, R2NH, R3N |
Classification by the type of organic reactions
Substitution Reactions
An atom or a group of atoms of the reactant molecule is replaced by another atom or group of atoms. Characteristic reactions of saturated compounds
e.g.
H2O
CH3
– Cl + NaOH
CH3
– OH + NaCl
Addition Reactions
Two molecules react to give a single product. Characteristic reactions of compounds with multiple bonds.
e.g.
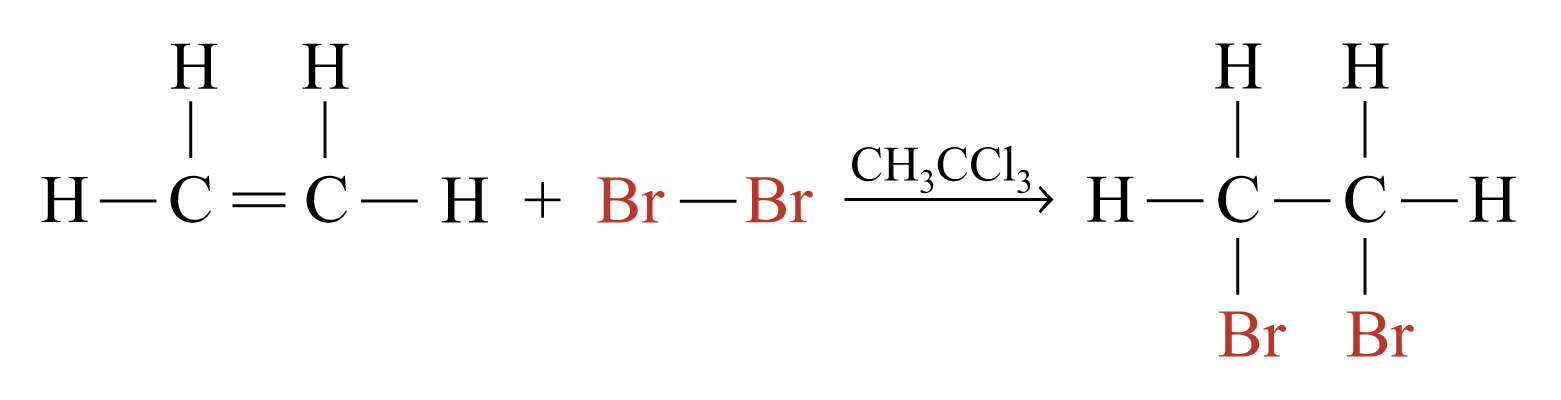
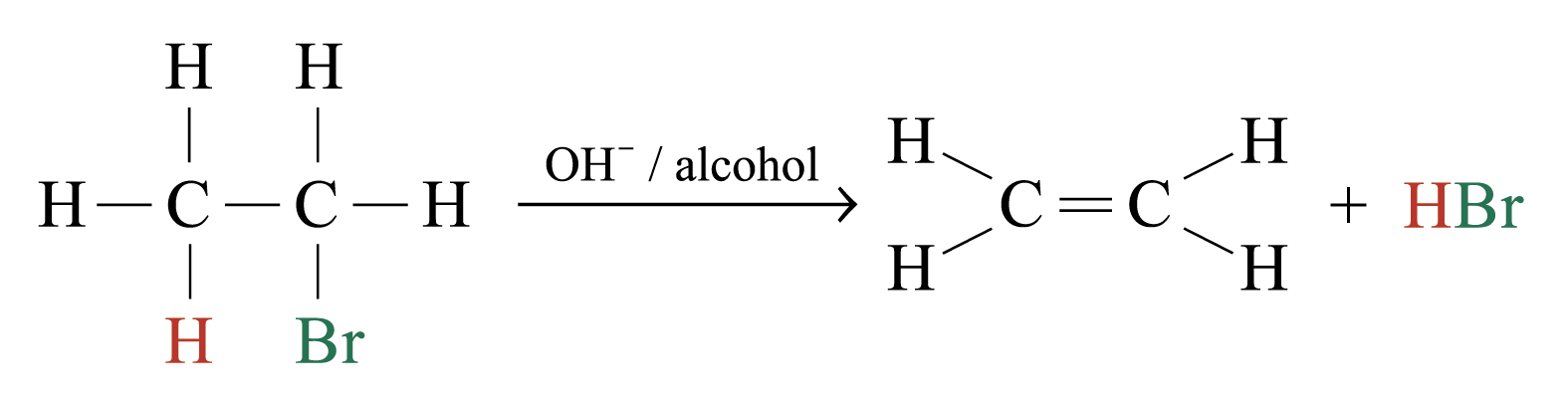
Elimination Reactions
Atoms or groups of atoms are removed from two adjacent atoms of the reactant molecule. Method for preparing compounds with multiple bonds.
e.g.
Condensation Reactions
Two or more molecules join together, with a small molecule being removed
e.g.
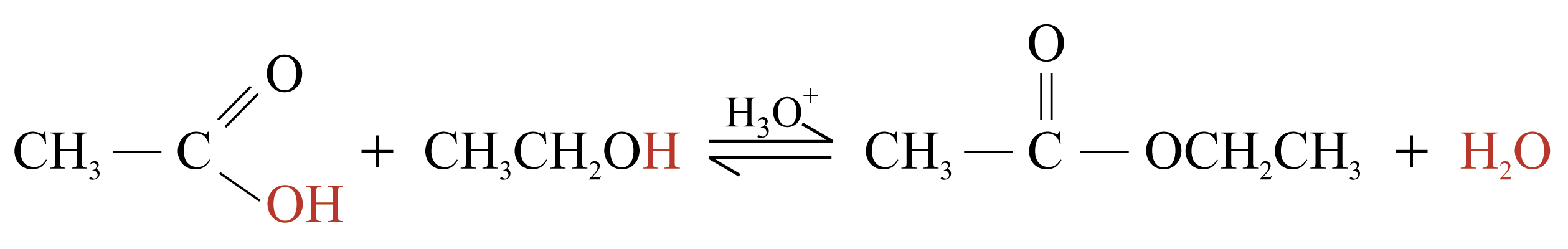
Rearrangement Reactions
A molecule undergoes reorganization of its constituent atoms or groups of atoms
e.g.

Classification by number of molecules, which take part in the slowest step of organic reaction (by order reaction)
Second-order reactions are more characteristic for organic chemistry.
e.g.
![]() - first-order
nucleophilic substitution reaction;
- first-order
nucleophilic substitution reaction;
![]() -
second-order
radical addition reaction.
-
second-order
radical addition reaction.
Isomerism
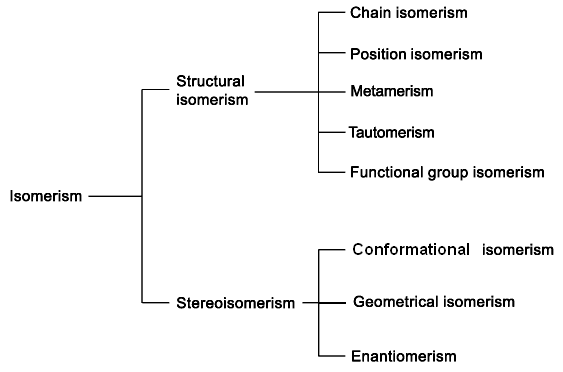
2.1. Structural Isomerism
Structural Isomers with the Same Functional Group
Chain Isomerism
Chain isomers are isomers that have different carbon skeletons. e.g.
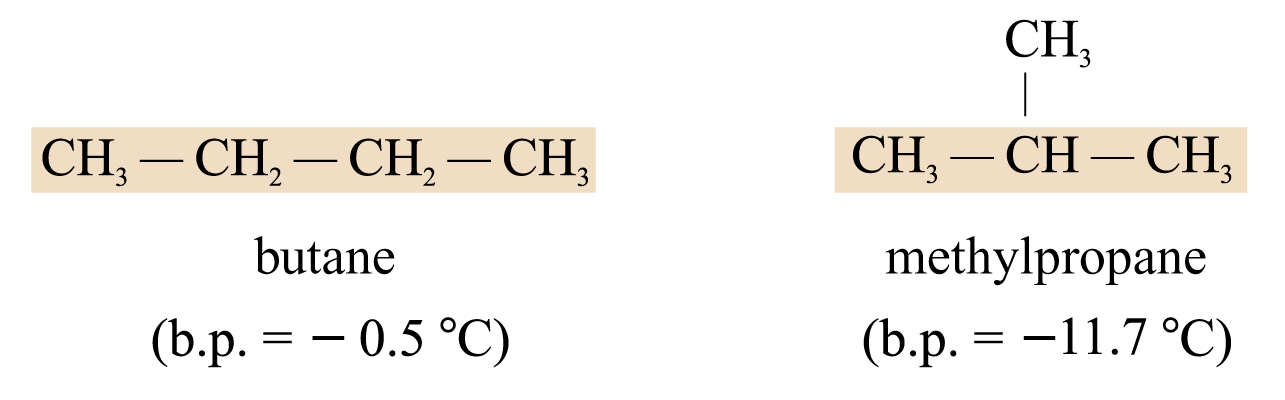

Position Isomerism
Position isomers are isomers that have the same carbon skeleton and functional group. They differ only in the position of the functional group. e.g.

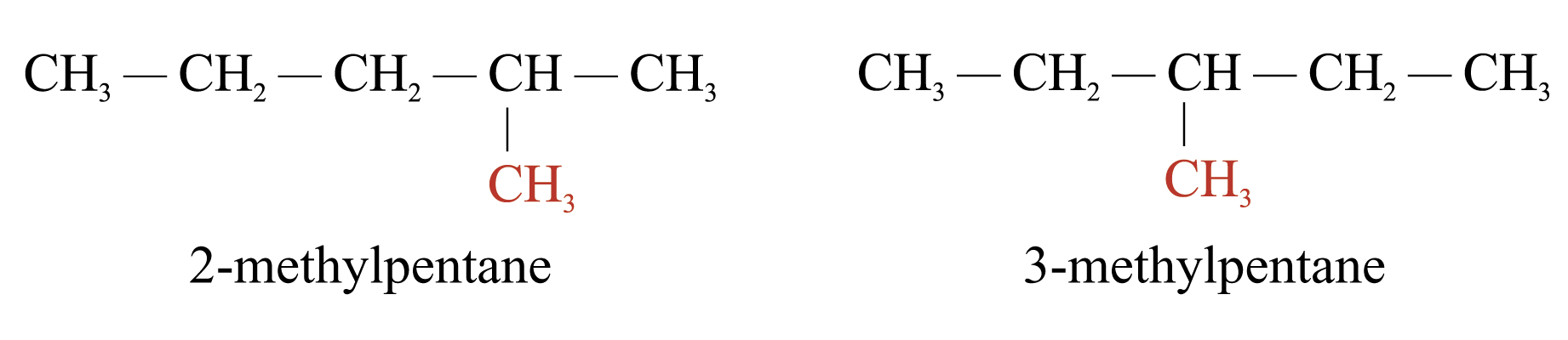
Metamerism
Metamers are those isomers with the functional group interrupting the carbon skeleton at different positions. e.g.
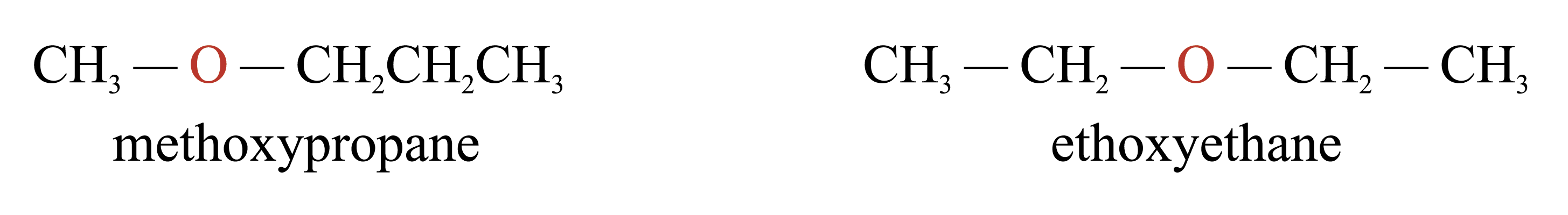
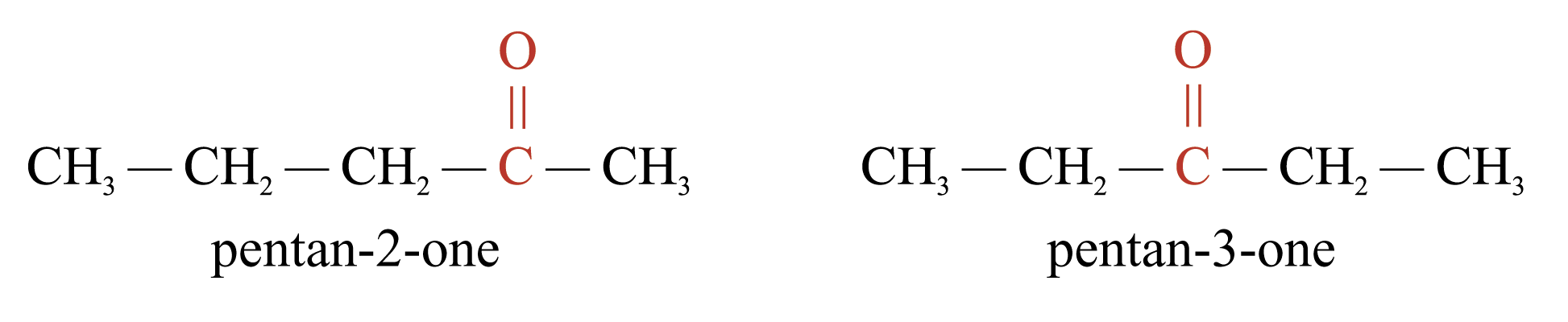
Tautomerism
Tautomers are those isomers with structures differing in arrangement of atoms. They are in dynamic equilibrium with each other. e.g.
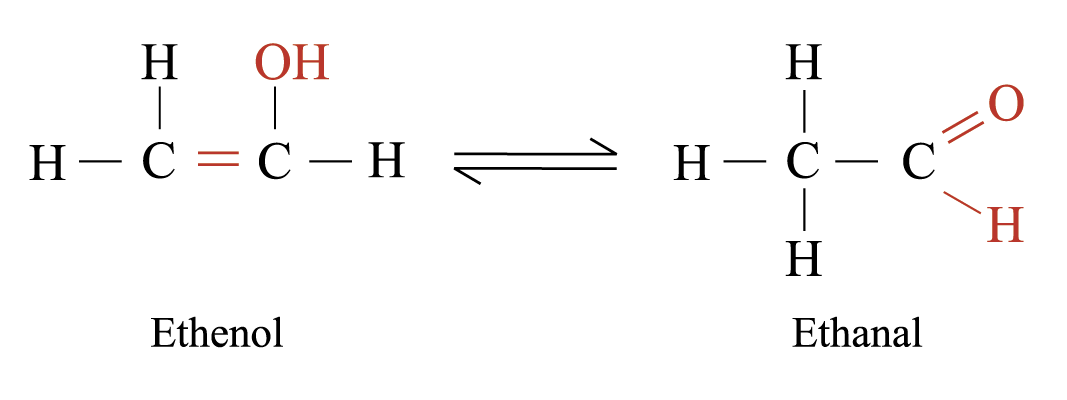
Structural Isomers with Different Functional Groups
Functional Group Isomerism
Functional group isomers are isomers that have the same molecular formula but contain different functional groups. e.g.
![]()

