
Occurrence Abundance of chalcogens in the Earth crust
|
S |
Se |
Te |
Po |
Abundance, by mass, % |
0.01 |
6.10-5 |
1.10-6 |
2.10-14 |
|
abundant |
scattered elements |
rare element |
|
sulfur is a relatively common element and occurs naturally as the element (in the caprock of salt domes and in the basin deposits in Poland), in metal sulfides (see Table below), and in sulfates such as gypsum. Furthermore, the sulfur deposits in natural gas and crude oil are now also becoming increasingly significant commercially. The world reserves of sulfur are believed to be in excess of 2 ∙ 109 tonnes.
Table. Some common sulfide minerals

Sulfate ores are: CaSО4.2H2O - gypsum, Na2SO4.10H2O is mirabilite, BaSO4 - baryte, MgSO4.7H2O - bitter salt et al.
Compounds of sulfur are also contained in combustible minerals, for example, in petroleum, admixtures of hydrogen sulfide are met in natural gas, sulfates – in saline water. Sulfur is a constituent part of biological tissues of all plants and animals.
In the plants sulfur accumulates, mainly, in the seeds and leaves. For example, cabbage has sulfur content about 0.8% (after conversion to the dry substance). Specific smells of garlic, mustard, onion and cabbage is caused by the organic compounds of sulfur. Animals have its especially large content in hair (to 4%), claws, horns and hoofs.
Sеlenium (content 6•10-5 % by mass) and tеllurium (1•10-6 % by mass) are dissipated elements, polonium (2•10-14 % by mass) is a rare element.
Selenium occurs naturally in a number of inorganic forms, including selenide, selenate, and selenite. Like sulfur, selenium is sometimes found in its elemental form. Native selenium is a rare mineral, which does not usually form good crystals (see figure below).

Native selenium
In soils, selenium most often occurs in soluble forms such as selenate (analogous to sulfate), which are leached into rivers very easily by runoff.
The ores of selenium are:
Berzelianite Cu2Se
Clausthalite PbSe (It forms a solid solution series with galena PbS).
Tetradimite Bi2Te2s
There are also selenites (chalcomenite CuSeО3•2Н2О) and selenates ( PbSeO4•2H2O).
The abundance of tellurium in earth’s crust is ca.1 0.01 ppm2. As to occurrence in the Earth's crust, tellurium is one of the most rare stable solid element (tellurium is sometimes found in its elemental form). Over 100 tellurium-containing minerals are known, but because of their low abundance, it is economically not viable to recover the element from its mineral ores.
It is worth noting that tellurium compounds are the most common chemical compounds of gold in nature. Although several gold deposits contain tellurium minerals nearly all tellurium is isolated in the process of copper and lead production as a by-product. Sе and Те can accompany elemental sulfur in nature.
In contrast to selenium, tellurium is not able to replace sulfur in its minerals. This is due to the large difference in ion radius of sulfur and tellurium. In consequence, many sulfide minerals contain considerable amounts of selenium, but only traces of tellurium
In the gold rush of 1893, diggers in Kalgoorlie discarded a pyritic material which got in their way as they searched for pure gold. The Kalgoorlie waste was thus used to fill in potholes or as part of sidewalks. Three years passed before it was realized that this waste was calaverite, a telluride of gold that had not been recognized. This led to a second gold rush in May 1896.
USES
About 50% of sulfur is spent on the production of H2SO4, 10-15%- as a pesticide against diseases of plants, sulfur is used for vulcanization in rubber industry, as a component of explosives, black gunpowder and in pharmaceutical industry.
Se and Te are important semiconductors. These materials are primarily used for making selenium rectifiers3, photocells (solar panels), photoresistors and the like. Selenium is applied as a pigment for glass and enamel (red color), in the process of vulcanization together with S, for making fine-grained structure of steel as a modifier. Tellurium is primarily used in alloys, foremost in steel and copper to improve machinability.
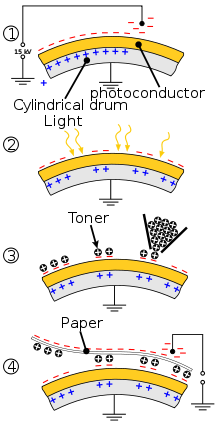

Scheme illustrating application of Se drums in Xeroxes
210Ро (-irradiator) is an energy source in the atomic heat sources of space satellites, in mixtures with Ве is used for making of ampoule sources of neutrons. When mixed or alloyed with beryllium, polonium can be a neutron source: beryllium releases a neutron upon absorption of an alpha particle that is supplied by 210Po. It has been used in this capacity as a neutron trigger or initiator for nuclear weapons. However, a license is needed to own and operate this form of neutron source
Due to its very high toxicity, polonium can be used as a poison (see, for example, Alexander Litvinenko poisoning).
