
- •Chrome subgroup physical properties
- •Chrome subgroup trends
- •History Of Discovery
- •Occurrence
- •Preparation
- •Electronic Configurations & Oxidation States
- •Chemical Properties. Free Chrome And Compounds (0)
- •Low oxidation states
- •Compounds e(IV)
- •Compounds e(V)
- •Questions and tasks
- •Make up the equations o f the reactions Make up the equations of the reactions
- •Experimental section
- •2. Chemical properties of chrome
- •3. Chemical properties to molybdenum and tungsten
- •Chrome. Standard electrode potentials, eo, V
- •Mendeleev's predicted elements
- •The Pourbaix diagram for chrome in pure water, perchloric acid or sodium hydroxide
- •M olybdenum Wheels
- •From Recent Developments in Inorganic Chemistry 2005
Electronic Configurations & Oxidation States
Chrome exhibits the wide range of oxidation states from -2 to +6. The outermost shells of chrome subgroup elements are: ...3d54s1(Cr), ...4d55s1(Mo), ...4f145d46s2(W). The excitement of the ground state of tungsten ...5d46s2 to ...5d56s1 state that is equivalent to the ground states of Cr and Mo requires about 33 kJ/mol.
In the series Cr-Mo-W electron shells are condensed and the ionisation energy is increased. Both Mo and W have high ionisation energy comparing with chrome and their chemical activity drops significantly. Chemical properties of molybdenum and tungsten are very similar.
-
Cr
Mo
W
Rа, nm
0,127
0,137
0,140
Ionic radius Е6+, nm
0,035
0,065
0,065
Ionisation energy Е Е+, eV
6,77
7,10
7,98
The most stable oxidation states of chrome are +3, +6. Molybdenum and tungsten have the most stable state +6 owing to closely-spased (n-1)d and ns sublevels. Compounds of Cr, Mo and W can also display oxidation states -2, -1, 0, +1, +2, +4, +5.
.
Chemical Properties. Free Chrome And Compounds (0)
Under ambient conditions, chrome is indifferent to water and air. Dilute sulfuric and hydrochloric acids dissolve chrome with evolving hydrogen. Reaction takes place with autoacceleration in consequence of destruction of the oxide film:
Cr + 2HCl = CrCl2 + H2.
Chrome does not dissolve in cold concentrated nitric acid and becomes passive after being treated with it. Molybdenum and tungsten don’t displace hydrogen. Molybdenum reacts gradually with HNO3:
Mo + 2HNO3 = H2MoO4 + 2NO
It is oxidised to Mo(VI) more rapidly with aqua regua, and HNO3 + H2SO4 mixture.
Tungsten does not react with acids whereas it can interact (like Mo) with HNO3 and HF mixture:
W + 8HF + 2HNO3 = H2[WF8] + 2NO + 4H2O
Mo and W can be oxidised by molten alkalis in the presence of oxidants:
2E + 4NaOH + 3O2 2Na2EO4 + 2H2O
or
Е + Na2CO3 + 3KNO3 = Na2ЕO4 + 3KNO2 + CO2
On being heated, Cr, Mo, W react with many non-metals:
4Cr + 3О2 = 2Cr2O3
(W) 2Mo + 3О2 = 2МоО3
Cr + 2F2 = CrF4
Mo(W) + 3F2 = Mo(W)F6
2Cr + 3Cl2 = 2CrCl3
2Mo + 5Cl2 = 2MoCl5
W + 3Cl2 = WCl6
and also with sulfur, nitrogen, carbon etc.
Scheme of chemical properties of chrome Low oxidation states of Cr,Oxidation state +2, Oxidation state +3, Oxidation state +4
Oxidation state +5
Oxidation state +6
The Pourbaix diagram for chrome
Low oxidation states
Compounds of chrome.
Oxides. Chrome forms three oxides: chrome(II) oxide CrO having a basic nature, chrome(III) oxide Cr2O3 exhibiting amphoteric properties, and chrome(VI) oxide or chromic anhydride CrO3— an acid oxide.
Scheme electron configuration of Ledovskix, Stepanenko
Oxidation state + 2
Chrome(II) Compounds. All compounds Cr(ІІ) display reducing properties. When chrome is dissolved in hydrochloric acid, a blue solution is obtained containing chromium(II) chloride CrCl2. If an alkali is added to this solution, a yellow precipitate of chrome(II) hydroxide Cr(OH)2 is formed.
CrCl2 + 2NaOH = Cr(OH)2 + 2NaCl
In turn, Cr(OH)2 is readily dissolved in acids. Cr2+ ions form deep blue aquocomplexes Cr(H2O)62+. All compounds of Cr(II) are strong reducing agents:
2CrCl2 + 2HCl = 2CrCl3 + H2
Сr(II) salts are also formed by CrHal3 (Hal = Cl, Br) reduction with H2 when heated:
2CrHal3 + H2 = 2CrHal2 + 2HHal
The compounds of chrome(II) are not stable and are rapidly oxidised by the oxygen of the air to chrome(III) compounds:
CrCl2 + О2 + 4HCl = 4CrCl3 + 2H2О.
They can even reduce water at the absence of an oxidising agent:
2CrCl2 + 2H2О = 2Cr(ОН)Cl2 + H2
Мо(II) and W(II) compounds are primarily halides МHal2 (Hal= Cl, Br, I). The latter can be produced by various methods. For instance, МоCl2 is produced by roasting metallic Mo in phosgene atmosphere:
Mo + COCl2 MoCl2 + CO
Мо(II) and W(II) compounds have unique structure. They contain groups of Mo(W) atoms having chemical bonds with one another. These unusual compounds with metal-metal bonds are called сlusters.
The group of six Мо atoms forms covalent bonds with six Cl atoms and four additional ionic bonds with Cl- are also created: [Mo6Cl8]4+Cl-4 = MoCl2. To form Mo-Mo bonds, each Мо atom spends four electrons whereas 4 free remaining orbitals are acceptors of electron pairs of donors in donor-acceptor covalent bonding with chlorine:
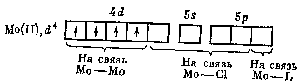
Mo-Mo bonds Mo-Cl bonds Mo –L bonds
The cluster [Mo6Cl8]4+ ion is rather stable. For instance, MoCl2 with actual composition [Mo6Cl8]4+Cl-4 forms the base [Mo6Cl8](ОН)4 with alkalis.
Oxidation state + 3
Compounds Е(ІІІ). Oxidation state +3 is the most stable state of chrome. Coordination number of Cr(III) is 6 that is why its complexes have octahedral shape. The number of compounds of Mo3+ and W3+ is insignificant.
Properties and structure of Cr(III) compounds in many respects are similar to compounds of aluminium that derives from identical size of their radii. This similarity manifests itself as hardness and amphoterity of Е2О3, Е(ОН)3, complete hydrolysis of Е2S3 and Е2(СО3)3 compounds. Chrome(III) salts resemble aluminium salts in many features. They become greatly hydrolyzed in aqueous solutions and readily transform into the basic salts. Chrome(III), like aluminium, forms no salts with weak acids.
Chrome(III) compounds. Chrome(III) oxide is a green refractory substance used under the name of chrome green to prepare oil paints. When fused with silicates, chrome (III) oxide colours them green and is therefore used for colouring glass and porcelain. This oxide is also a component of polishing compounds.
Сr2O3 is usually a dark green powder or sometimes black crystals with metallic luster. Like Al2O3 Cr(III) oxide has polymeric structure of corundum that defines refractory properties (t0m = 22750С) and hardness (Mohs hardness 8-8.5) of Cr2O3. On the other hand, this material is brittle and antiferromagnetic up to 307 K.
Cr2O3 is obtained by decomposition reactions, for instance:
2Cr(OH)3 = Cr2O3 + 3H2O
(NH4)2Cr2O7 = Cr2O3 + N2 + 4H2O
Cr2O3 is chemically inert: insoluble in water, acids, and alkalis. Its amphoteric properties become apparent when melting with alkalis and basic oxides (chromites are formed):
Cr2O3 + 6NaOH = Na3CrO3 + 3H2O
Basic properties are realised by the reactions:
Cr2O3 + 3K2S2O7 = Cr2(SO4)3 + 3K2SO4
Cr2O3 + 6NaHSO4 = Cr2(SO4)3 + 3Na2SO4 + 3H2O
Cr3+ is oxidised to Cr(VI) in alkaline medium, particularly when fused Cr2O3 with KOH + KClO3 or Na2CO3 + KNO3 mixtures:
Cr2O3 + 3KNO3 + 2Na2CO3 = 2Na2CrO4 + 3KNO2 + 2CO2
Chrome(III) hydroxide Cr(OH)3 forms a bluish gray precipitate when alkalis react with a chrome(III) salt:
Сr3+ + 3OH- = Cr(OH)3
The actual mechanism of this process: [Cr(H2O)6]3+ aqueocomplex have the tendency to the polycondensation process as a result of step-by-step transformation into hydroxoaqueocomplexes [Cr(H2O)5ОН]2+, [Cr(H2O)4(ОН)2]+, and [Cr(H2O)6(ОН)3], respectively. It can be summated by the reaction:
[Cr(H2O)6]3+ + 3ОН- = [Cr(H2O)3(ОН)3] + 3Н2О
The neutral [Cr(H2O)3(ОН)3] coordination compound sufficiently decreases repulsion forces between particles and polycondensation process takes place through the Cr-ОН-Cr bridges. The structure of such polymer is shown below:
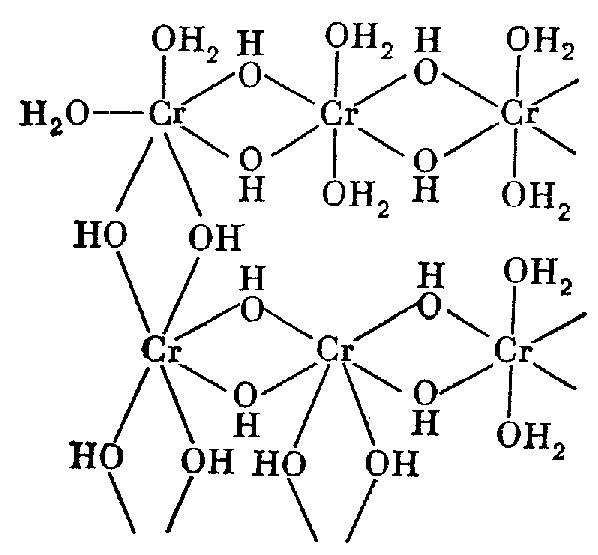
Chrome (III) hydroxide of gray-blue colour has nonstoichiometric composition Cr2O3.nH2O, therefore formula Cr(OH)3 is arbitrary.
Like aluminium and zinc hydroxides, it is amphoteric and is dissolved in acids to form chrome(III) salts:
Cr(OH)3 + 3H3O+ [Cr(H2O)6]3+
and in alkalis to form emerald green solutions of chromites:
Cr(OH)3 + 3NaOH = Na3[Cr(OH)6]
or
Cr(OH)3 + 3OH- = [Cr(OH)6]3-
The chemical activity of Cr(OH)3 is decreased as a result of gradual displacement of Cr-OH-Cr bonds by Cr-O-Cr bridges. The equilibrium state between Cr3+ hydroxo- and aquocomplexes can be shown as follows:

acid medium neutral medium alkaline medium
Chrome(III) salts. Chromites prepared by fusing Cr2O3 with oxides of other metals are known chiefly for divalent metals. They have a composition expressed by the formula M(CrO2)2 including the natural chromite Fe(CrO2)2.
Solutions of chrome(III) salts generally have a bluish violet colour, but turn green when heated. This colour change is explained by the formation of isomeric salt hydrates that are coordination compounds in which all or part of the water molecules are coordinationally bonded in the inner sphere of the complex. For instance, the crystal hydrate of chrome(III) chloride CrCl3-6H2O is known in three isomeric forms—bluish violet, dark green, and light green crystals having an identical composition. The structure of these isomers can be established on the basis of the different reaction of the freshly prepared solutions with silver nitrate. When the latter reacts with a solution of the bluish violet hydrate, all the chlorine precipitates; two-thirds of the chlorine precipitate from a solution of the dark green hydrate, and only one-third from a solution of the light green hydrate. Taking into account these data, and also the coordination number of chrome equal to six, the structure of these crystal hydrates can be expressed by the following formulas:
[Cr(H2O)6]Cl3 [Cr(H2O)5Cl]Cl2.H2O [Cr(H2O)4Cl2]Cl.2H2O bluish violet dark green light green
t oC

Hence, the isomerism of the chrome(III) chloride hydrates is due to the different distribution of the same groups (H2O and Cl-) between the inner and outer coordination spheres and can be an example of hydrate isomerism.
The transformations of CrCl3.6H2O complex ion in aqueous solution are outlined below:

Oxidation state + 3
Cr(ІІІ) salts that are isolated from aqueous solutions are always crystalhydrates: Cr(NO3)3•9H2O, CrCl3•6H2O and etc. Chrome can form alums КCr(SO4)2•12H2O. They have double salt structure [M(H2O)6]•[Cr(H2O)6](SO4)2.
Cr(III) reveals large number of coordination compounds with different ligands. They all have octahedral structure that is defined by 3d54s14p0 configuration. At first, chrome atom loses electron from the outermost 4s-subshell, and then from the preceding 3d-subshell. The following electron configuration of Cr(III) is formed: 3d34s04p0.
 [Cr(H2O)6]3+
[Cr(H2O)6]3+
![]()
Two free 3d-orbitals, single 4s-orbital and three 4р-orbitals participate in donor-acceptor bonds formation with Н2О molecules (d2sp3-hybridisation). Thus, [Cr(H2O)6]3+ ion is an example of innerorbital complex ion.
The interesting feature of coordination compounds of Cr(III) is their low lability, i.e. slow displacement rate of ligands in the inner sphere. The reason is the presence of three unpaired d-electrons of chrome with high energy of octahedral crystal field stabilisation. Moreover, the unpaired d-electrons define paramagnetic properties and colours. The low lability allows to synthesize the significant number of various coordination compounds even those compounds that are thermodynamically unstable.
Aquocomplexes [Cr(H2O)6]3+ are stable in solutions and also in the solid state forming crystalhydrates (CrCl3.6H2O, KСr(SO4)2.12H2O etc.).
Cr(III) salts are usually hydrolysed in aqueous solutions. The first step of hydrolysis is:
[Cr(H2O)6]3+ + H2O = [Cr(OH)(H2O)5]2+ + H3O+
or Cr3+ + H2O = Cr(OH)2+ + H+
Salts of weak acids hydrolyse completely, therefore:
2Cr(NO3)3 + 3Na2CO3 + 3H2O = 2Cr(OH)3 + 6NaNO3 + 3CO2
The reaction mechanism with Na2SiO3, Na2S, Na2B4O7 is also similar.
Red-Ox properties. Cr(III) reacts mostly with strong oxidising and reducing agents.
Cr(III) oxidising properties:
Cr2O3 + 2Al = 2Cr + Al2O3
2CrCl3 + Zn = 2CrCl2 + ZnCl2
Cr(III) reducing properties. In the presence of oxidants in melts:
2Cr2O3 + 8NaOH + 3O2 = 4Na2CrO4 + 4H2O (melting)
Cr2O3 + 2NaCO3 + 3NaNО3 = 2Na2CrO4 + 3NaNO2 + 2CO2
Halogens (Cl2, Br2), H2O2 and some other oxidising agents are applied in alkaline medium:
2Сr(NO3)3 + 3Cl2 + 16NaOH = 2Na2CrO4 + 6NaCl + 6NaNO3 + 8H2O
whereas even stronger oxidising agents (peroxosulfate, ozone etc.) are utilised in acid and neutral mediums:
2Cr(NО3)3
+ 3K2S2O8
+ 7H2O
![]() H2Cr2О7
+ 3K2SO4
+ 3H2SO4
+ 6HNO3
H2Cr2О7
+ 3K2SO4
+ 3H2SO4
+ 6HNO3
Cr2(SО4)3
+ 3O3
+ 4H2O
![]() H2Cr2О7
+ 3H2SO4
+ 3O2
H2Cr2О7
+ 3H2SO4
+ 3O2
Cr2(SO4)3 + 3(NH4)2S2O8 + 7H2O = H2Cr2O7 + 3(NH4)2SO4 + 6H2SO4
Mo(ІІІ) and W(ІІІ) compounds are rare:
МоF6 + Mo 2МоF3
МоCl5 + H2 = МоCl3 + 2HCl
