
- •Foreword
- •Preface
- •Contents
- •About the Editors
- •Contributors
- •1: Tracheobronchial Anatomy
- •Trachea
- •Introduction
- •External Morphology
- •Internal Morphology
- •Mucous Layer
- •Blood Supply
- •Anatomo-Clinical Relationships
- •Bronchi
- •Main Bronchi
- •Bronchial Division
- •Left Main Bronchus (LMB)
- •Right Main Bronchus (RMB)
- •Blood Supply
- •References
- •2: Flexible Bronchoscopy
- •Introduction
- •History
- •Description
- •Indications and Contraindications
- •Absolute Contraindications
- •Procedure Preparation
- •Technique of FB Procedure
- •Complications of FB Procedure
- •Basic Diagnostic Procedures
- •Bronchoalveolar Lavage (BAL)
- •Transbronchial Lung Biopsy (TBLB)
- •Transbronchial Needle Aspiration (TBNA)
- •Bronchial Brushings
- •Advanced Diagnostic Bronchoscopy
- •EBUS-TBNA
- •Ultrathin Bronchoscopy
- •Transbronchial Lung Cryobiobsy (TBLC)
- •Therapeutic Procedures Via FB
- •LASER Bronchoscopy
- •Electrocautery
- •Argon Plasma Coagulation (APC)
- •Cryotherapy
- •Photodynamic Therapy
- •Airway Stent Placement
- •Endobronchial Valve Placement
- •Conclusion
- •References
- •History and Historical Perspective
- •Indications and Contraindications
- •Procedure Description
- •Procedure Planning
- •Target Approximation
- •Sampling
- •Complications
- •Future Directions
- •Summary and Recommendations
- •References
- •4: Rigid Broncoscopy
- •Innovations
- •Ancillary Equipment
- •Rigid Bronchoscopy Applications
- •Laser Bronchoscopy
- •Tracheobronchial Prosthesis
- •Transbronchial Needle Aspiration (TBNA)
- •Rigid Bronchoscope in Other Treatments for Bronchial Obstruction
- •Mechanical Debridement
- •Pediatric Rigid Bronchoscopy
- •Tracheobronchial Dilatation
- •Foreign Bodies Removal
- •Other Indications
- •Complications
- •The Procedure
- •Some Conclusions
- •References
- •History and Historical Perspective
- •Indications and Contraindications
- •Preprocedural Evaluation and Preparation
- •Physical Examination
- •Procedure-Related Indications
- •Application of the Technique
- •Topical Anesthesia
- •Anesthesia of the Nasal Mucosa and Nasopharynx
- •Anesthesia of the Mouth and Oropharynx
- •Superior Laryngeal Nerve Block
- •Recurrent Laryngeal Nerve Block (RLN)
- •Conscious Sedation
- •Monitored Anesthesia Care (MAC)
- •General Anesthesia
- •Monitoring the Depth of Anesthesia
- •Interventional Bronchoscopy Suites
- •Airway Devices
- •Laryngeal Mask Airway (LMA)
- •Endotracheal Tube (ETT)
- •Rigid Bronchoscope
- •Modes of Ventilation
- •Spontaneous Ventilation
- •Assisted Ventilation
- •Noninvasive Positive Pressure Ventilation (NIV)
- •Positive Pressure Controlled Mechanical Ventilation
- •Jet Ventilation
- •Electronic Mechanical Jet Ventilation
- •Postprocedure Care
- •Special Consideration
- •Anesthesia for Peripheral Diagnostic and Therapeutic Bronchoscopy
- •Anesthesia for Interventional Bronchoscopic Procedures During the COVID-19 Pandemic
- •Summary and Recommendations
- •Conclusion
- •References
- •Background
- •Curricular Structure and Delivery
- •What Is a Bronchoscopy Curriculum?
- •Tradition, Teaching Styles, and Beliefs
- •Using Assessment Tools to Guide the Educational Process
- •The Ethics of Teaching
- •When Learners Teach: The Journey from Novice to Mastery and Back Again
- •The Future Is Now
- •References
- •Interventional Procedure
- •Assessment of Flow–Volume Curve
- •Dyspnea
- •Analysis of Pressure–Pressure Curve
- •Conclusions
- •References
- •Introduction
- •Adaptations of the IP Department
- •Environmental Control
- •Personal Protective Equipment
- •Procedure Performance
- •Bronchoscopy in Intubated Patients
- •Other Procedures in IP Unit
- •References
- •Introduction
- •Safety
- •Patient Safety
- •Provider Safety
- •Patient Selection and Screening
- •Lung Cancer Diagnosis and Staging
- •Inpatients
- •COVID-19 Clearance
- •COVID Clearance: A Role for Bronchoscopy
- •Long COVID: A Role for Bronchoscopy
- •Preparing for the Next Pandemic
- •References
- •Historical Perspective
- •Indications and Contraindications
- •Evidence-Based Review
- •Summary and Recommendations
- •References
- •Introduction
- •Clinical Presentation
- •Diagnosis
- •Treatment
- •History and Historical Perspectives
- •Indications and Contraindications
- •Benign and Malignant Tumors
- •Tumors with Uncertain Prognosis
- •Application of the Technique
- •Evidence Based Review
- •Summary and Recommendations
- •References
- •12: Cryotherapy and Cryospray
- •Introduction
- •Historical Perspective
- •Equipment
- •Cryoadhesion
- •Indications
- •Cryorecanalization
- •Cryoadhesion and Foreign Body Removal
- •Cryoadhesion and Mucus Plugs/Blood Clot Retrieval
- •Endobronchial Cryobiopsy
- •Transbronchial Cryobiopsy for Lung Cancer
- •Safety Concerns and Contraindications
- •Cryoablation
- •Indications
- •Evidence
- •Safety Concerns and Contraindications
- •Cryospray
- •Indications
- •Evidence
- •Safety Concerns and Contraindications
- •Advantages of Cryotherapy
- •Limitations
- •Future Research Directions
- •References
- •13: Brachytherapy
- •History and Historical Perspective
- •Indications and Contraindications
- •Application of the Technique
- •Evidence-Based Review
- •Adjuvant Treatment
- •Palliative Treatment
- •Complications
- •Summary and Recommendations
- •References
- •14: Photodynamic Therapy
- •Introduction
- •Photosensitizers
- •First-Generation Photosensitizers
- •M-Tetrahidroxofenil Cloro (mTHPC) (Foscan®)
- •PDT Reaction
- •Tumor Damage Process
- •Procedure
- •Indications
- •Curative PDT Indications
- •Palliative PDT Indications
- •Contraindications
- •Rationale for Use in Early-Stage Lung Cancer
- •Rationale
- •PDT in Combination with Other Techniques for Advanced-Stage Non-small Cell Lung Cancer
- •Commentary
- •Complementary Endoscopic Methods for PDT Applications
- •New Perspectives
- •Other PDT Applications
- •Conclusions
- •References
- •15: Benign Airways Stenosis
- •Etiology
- •Congenital Tracheal Stenosis
- •Iatrogenic
- •Infectious
- •Idiopathic Tracheal Stenosis
- •Distal Bronchial Stenosis
- •Diagnosis Methods
- •Patient History
- •Imaging Techniques
- •Bronchoscopy
- •Pulmonary Function Test
- •Treatment
- •Endoscopic Treatment
- •Dilatation
- •Laser Therapy
- •Stents
- •How to Proceed
- •Stent Placement
- •Placing a Montgomery T Tube
- •The Rule of Twos for Benign Tracheal Stenosis (Fig. 15.23)
- •Surgery
- •Summary and Recommendations
- •References
- •16: Endobronchial Prostheses
- •Introduction
- •Indications
- •Extrinsic Compression
- •Intraluminal Obstruction
- •Stump Fistulas
- •Esophago-respiratory Fistulas (ERF)
- •Expiratory Central Airway Collapse
- •Physiologic Rationale for Airway Stent Insertion
- •Stent Selection Criteria
- •Stent-Related Complications
- •Granulation Tissue
- •Stent Fracture
- •Migration
- •Contraindications
- •Follow-Up and Patient Education
- •References
- •Introduction
- •Overdiagnosis
- •False Positives
- •Radiation
- •Risk of Complications
- •Lung Cancer Screening Around the World
- •Incidental Lung Nodules
- •Management of Lung Nodules
- •References
- •Introduction
- •Minimally Invasive Procedures
- •Mediastinoscopy
- •CT-Guided Transthoracic Biopsy
- •Fluoroscopy-Guided Transthoracic Biopsies
- •US-Guided Transthoracic Biopsy
- •Thoracentesis and Pleural Biopsy
- •Thoracentesis
- •Pleural Biopsy
- •Surgical or Medical Thoracoscopy
- •Image-Guided Pleural Biopsy
- •Closed Pleural Biopsy
- •Image-Guided Biopsies for Extrathoracic Metastases
- •Tissue Acquisition, Handling and Processing
- •Implications of Tissue Acquisition
- •Guideline Recommendations for Tissue Acquisition in Mediastinal Staging
- •Methods to Overcome Challenges in Tissue Acquisition and Genotyping
- •Rapid on-Site Evaluation (ROSE)
- •Sensitive Genotyping Assays
- •Liquid Biopsy
- •Summary, Recommendations and Highlights
- •References
- •History
- •Data Source and Methodology
- •Tumor Size
- •Involvement of the Main Bronchus
- •Atelectasis/Pneumonitis
- •Nodal Staging
- •Proposal for the Revision of Stage Groupings
- •Small Cell Lung Cancer (SCLC)
- •Discussion
- •Methodology
- •T Descriptors
- •N Descriptors
- •M Descriptors
- •Summary
- •References
- •Introduction
- •Historical Perspective
- •Fluoroscopy
- •Radial EBUS Mini Probe (rEBUS)
- •Ultrasound Bronchoscope (EBUS)
- •Virtual Bronchoscopy
- •Trans-Parenchymal Access
- •Cone Beam CT (CBCT)
- •Lung Vision
- •Sampling Instruments
- •Conclusions
- •References
- •History and Historical Perspective
- •Narrow Band Imaging (NBI)
- •Dual Red Imaging (DRI)
- •Endobronchial Ultrasound (EBUS)
- •Optical Coherence Tomography (OCT)
- •Indications and Contraindications
- •Confocal Laser Endomicroscopy and Endocytoscopy
- •Raman Spectrophotometry
- •Application of the Technique
- •Supplemental Technology for Diagnostic Bronchoscopy
- •Evidence-Based Review
- •Summary and Recommendations, Highlight of the Developments During the Last Three Years (2013 on)
- •References
- •Introduction
- •History and Historical Perspective
- •Endoscopic AF-OCT System
- •Preclinical Studies
- •Clinical Studies
- •Lung Cancer
- •Asthma
- •Airway and Lumen Calibration
- •Obstructive Sleep Apnea
- •Future Applications
- •Summary
- •References
- •23: Endobronchial Ultrasound
- •History and Historical Perspective
- •Equipment
- •Technique
- •Indication, Application, and Evidence
- •Convex Probe Ultrasound
- •Equipment
- •Technique
- •Indication, Application, and Evidence
- •CP-EBUS for Malignant Mediastinal or Hilar Adenopathy
- •CP-EBUS for the Staging of Non-small Cell Lung Cancer
- •CP-EBUS for Restaging NSCLC After Neoadjuvant Chemotherapy
- •Complications
- •Summary
- •References
- •Introduction
- •What Is Electromagnetic Navigation?
- •SuperDimension Navigation System (EMN-SD)
- •Computerized Tomography
- •Computer Interphase
- •The Edge Catheter: Extended Working Channel (EWC)
- •Procedural Steps
- •Planning
- •Detecting Anatomical Landmarks
- •Pathway Planning
- •Saving the Plan and Exiting
- •Registration
- •Real-Time Navigation
- •SPiN System Veran Medical Technologies (EMN-VM)
- •Procedure
- •Planning
- •Navigation
- •Biopsy
- •Complications
- •Limitations
- •Summary
- •References
- •Introduction
- •Image Acquisition
- •Hardware
- •Practical Considerations
- •Radiation Dose
- •Mobile CT Studies
- •Future Directions
- •Conclusion
- •References
- •26: Robotic Assisted Bronchoscopy
- •Historical Perspective
- •Evidence-Based Review
- •Diagnostic Yield
- •Monarch RAB
- •Ion Endoluminal Robotic System
- •Summary
- •References
- •History and Historical Perspective
- •Indications and Contraindications
- •General
- •Application of the Technique
- •Preoperative Care
- •Patient’s Position and Operative Field
- •Incision and Initial Dissection
- •Palpation
- •Biopsy
- •Control of Haemostasis and Closure
- •Postoperative Care
- •Complications
- •Technical Variants
- •Extended Cervical Mediastinoscopy
- •Mediastinoscopic Biopsy of Scalene Lymph Nodes
- •Inferior Mediastinoscopy
- •Mediastino-Thoracoscopy
- •Video-Assisted Mediastinoscopic Lymphadenectomy
- •Transcervical Extended Mediastinal Lymphadenectomy
- •Evidence-Based Review
- •Summary and Recommendations
- •References
- •Introduction
- •Case 1
- •Adrenal and Hepatic Metastases
- •Brain
- •Bone
- •Case 1 Continued
- •Biomarkers
- •Case 1 Concluded
- •Case 2
- •Chest X-Ray
- •Computerized Tomography
- •Positive Emission Tomography
- •Magnetic Resonance Imaging
- •Endobronchial Ultrasound with Transbronchial Needle Aspiration
- •Transthoracic Needle Aspiration
- •Transbronchial Needle Aspiration
- •Endoscopic Ultrasound with Needle Aspiration
- •Combined EUS-FNA and EBUS-TBNA
- •Case 2 Concluded
- •Case 3
- •Standard Cervical Mediastinoscopy
- •Extended Cervical Mediastinoscopy
- •Anterior Mediastinoscopy
- •Video-Assisted Thoracic Surgery
- •Case 3 Concluded
- •Case 4
- •Summary
- •References
- •29: Pleural Anatomy
- •Pleural Embryonic Development
- •Pleural Histology
- •Cytological Characteristics
- •Mesothelial Cells Functions
- •Pleural Space Defense Mechanism
- •Pleura Macroscopic Anatomy
- •Visceral Pleura (Pleura Visceralis or Pulmonalis)
- •Parietal Pleura (Pleura Parietalis)
- •Costal Parietal Pleura (Costalis)
- •Pleural Cavity (Cavitas Thoracis)
- •Pleural Apex or Superior Pleural Sinus [12–15]
- •Anterior Costal-Phrenic Sinus or Cardio-Phrenic Sinus
- •Posterior Costal-Phrenic Sinus
- •Cost-Diaphragmatic Sinus or Lateral Cost-Phrenic Sinus
- •Fissures18
- •Pleural Vascularization
- •Parietal Pleura Lymphatic Drainage
- •Visceral Pleura Lymphatic Drainage
- •Pleural Innervation
- •References
- •30: Chest Ultrasound
- •Introduction
- •The Technique
- •The Normal Thorax
- •Chest Wall Pathology
- •Pleural Pathology
- •Pleural Thickening
- •Pneumothorax
- •Pulmonary Pathology
- •Extrathoracic Lymph Nodes
- •COVID and Chest Ultrasound
- •Conclusions
- •References
- •Introduction
- •History of Chest Tubes
- •Overview of Chest Tubes
- •Contraindications for Chest Tube Placement
- •Chest Tube Procedural Technique
- •Special Considerations
- •Pneumothorax
- •Empyema
- •Hemothorax
- •Chest Tube Size Considerations
- •Pleural Drainage Systems
- •History of and Introduction to Indwelling Pleural Catheters
- •Indications and Contraindications for IPC Placement
- •Special Considerations
- •Non-expandable Lung
- •Chylothorax
- •Pleurodesis
- •Follow-Up and IPC Removal
- •IPC-Related Complications and Management
- •Competency and Training
- •Summary
- •References
- •32: Empyema Thoracis
- •Historical Perspectives
- •Incidence
- •Epidemiology
- •Pathogenesis
- •Clinical Presentation
- •Radiologic Evaluation
- •Biochemical Analysis
- •Microbiology
- •Non-operative Management
- •Prognostication
- •Surgical Management
- •Survivorship
- •Summary and Recommendations
- •References
- •Evaluation
- •Initial Intervention
- •Pleural Interventions for Recurrent Symptomatic MPE
- •Especial Circumstances
- •References
- •34: Medical Thoracoscopy
- •Introduction
- •Diagnostic Indications for Medical Thoracoscopy
- •Lung Cancer
- •Mesothelioma
- •Other Tumors
- •Tuberculosis
- •Therapeutic Indications
- •Pleurodesis of Pneumothorax
- •Thoracoscopic Drainage
- •Drug Delivery
- •Procedural Safety and Contraindications
- •Equipment
- •Procedure
- •Pre-procedural Preparations and Considerations
- •Procedural Technique [32]
- •Medical Thoracoscopy Versus VATS
- •Conclusion
- •References
- •Historical Perspective
- •Indications and Contraindications
- •Evidence-Based Review
- •Endobronchial Valves
- •Airway Bypass Tracts
- •Coils
- •Other Methods of ELVR
- •Summary and Recommendations
- •References
- •36: Bronchial Thermoplasty
- •Introduction
- •Mechanism of Action
- •Trials
- •Long Term: Ten-Year Study
- •Patient Selection
- •Bronchial Thermoplasty Procedure
- •Equipment
- •Pre-procedure
- •Bronchoscopy
- •Post-procedure
- •Conclusion
- •References
- •Introduction
- •Bronchoalveolar Lavage (BAL)
- •Technical Aspects of BAL Procedure
- •ILD Cell Patterns and Diagnosis from BAL
- •Technical Advises for Conventional TLB and TLB-C in ILD
- •Future Directions
- •References
- •Introduction
- •The Pediatric Airway
- •Advanced Diagnostic Procedures
- •Endobronchial Ultrasound
- •Virtual Navigational Bronchoscopy
- •Cryobiopsy
- •Therapeutic Procedures
- •Dilation Procedures
- •Thermal Techniques
- •Mechanical Debridement
- •Endobronchial Airway Stents
- •Metallic Stents
- •Silastic Stents
- •Novel Stents
- •Endobronchial Valves
- •Bronchial Thermoplasty
- •Discussion
- •References
- •Introduction
- •Etiology
- •Congenital ADF
- •Malignant ADF
- •Cancer Treatment-Related ADF
- •Benign ADF
- •Iatrogenic ADF
- •Diagnosis
- •Treatment Options
- •Endoscopic Techniques
- •Stents
- •Clinical Results
- •Stent Complications
- •Other Available Stents
- •Other Endoscopic Methods
- •References
- •Introduction
- •Anatomy and Physiology of Swallowing
- •Functional Physiology of Swallowing
- •Epidemiology and Risk Factors
- •Types of Foreign Bodies
- •Organic
- •Inorganic
- •Mineral
- •Miscellaneous
- •Clinical Presentation
- •Acute FB
- •Retained FB
- •Radiologic Findings
- •Bronchoscopy
- •Airway Management
- •Rigid Vs. Flexible Bronchoscopy
- •Retrieval Procedure
- •Instruments
- •Grasping Forceps
- •Baskets
- •Balloons
- •Suction Instruments
- •Ablative Therapies
- •Cryotherapy
- •Laser Therapy
- •Electrocautery and APC
- •Surgical Management
- •Complications
- •Bleeding and Hemoptysis
- •Distal Airway Impaction
- •Iron Pill Aspiration
- •Follow-Up and Sequelae
- •Conclusion
- •References
- •Vascular Origin of Hemoptysis
- •History and Historical Perspective
- •Diagnostic Bronchoscopy
- •Therapeutic Bronchoscopy
- •General Measures
- •Therapeutic Bronchoscopy
- •Evidence-Based Review
- •Summary
- •Recommendations
- •References
- •History
- •“The Glottiscope” (1807)
- •“The Esophagoscope” (1895)
- •The Rigid Bronchoscope (1897–)
- •The Flexible Bronchoscope (1968–)
- •Transbronchial Lung Biopsy (1972) (Fig. 42.7)
- •Laser Therapy (1981–)
- •Endobronchial Stents (1990–)
- •Electromagnetic Navigation (2003–)
- •Bronchial Thermoplasty (2006–)
- •Endobronchial Microwave Therapy (2004–)
- •American Association for Bronchology and Interventional Pulmonology (AABIP) and Journal of Bronchology and Interventional Pulmonology (JOBIP) (1992–)
- •References
- •Index
590 |
C. A. Jiménez and V. R. Shannon |
|
|
Management of Malignant Pleural
E usions
Evaluation
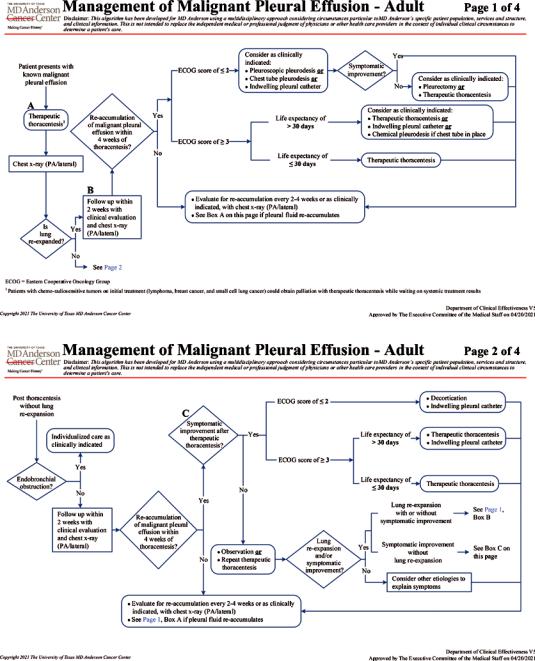
Because MPE often signals advanced disease and incurability, the goals of care are usually aimed at symptom palliation. A complete history and physical evaluation are key in determining individual palliative treatment goals. Selecting the best therapeutic option is based on the patient’s exercise tolerance and ability to perform certain activities of daily living (performance status), tumor histology, and estimated life expectancy. Information regarding prior thoracenteses, including the volume of uid evacuated, whether lung re-expansion and symptom palliation were obtained, and the time interval between repeated taps, is a key component of the initial evaluation. Predicting the life span of individual patients is challenging. Certain prognostic tools (i.e., LENT and PROMISE) are seldom used in clinical practice and often the prediction is made using the performance status and clinical information [36– 38]. Chest wall abnormalities, cancer treatment plans, the patient’s beliefs and expectations, and the availability of family support should be pondered before proposing a strategy. Figure 33.5a, b shows the algorithms for management of MPE at our institution [39].
Initial Intervention
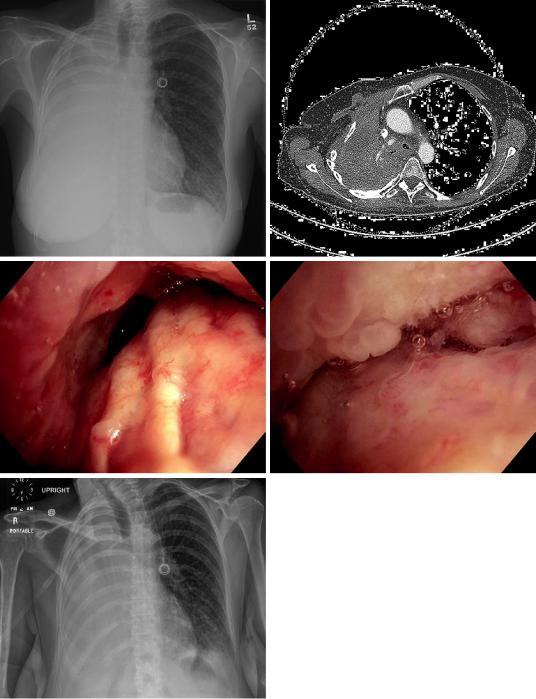
Since most of the individuals with a suspected or stablished MPE will most likely require a thoracentesis, we recommend performing an ultrasound-guided symptom-limited maximal drainage of uid using any of the commercially available custom-ft safety thoracentesis needles with small-bore catheters. This approach may be applied even on to those patients with ipsilateral deviation of the mediastinum (Fig. 33.6a–e) [40]. Patients can safely tolerate
more than 1.5 L of uid drainage in one sitting as long as there are no procedure-related symptoms of chest pain/discomfort or persistent cough. It is desirable to have a diagnostic image of the chest within 2–3 days prior to the intervention to compare it with a post-procedure one. Evidence for the use of pleural manometry during large volume thoracentesis is limited and its implementation is cumbersome, time consuming, and potentially costly. Additionally, poor implementation and interpretation of the data can lead to unexpected harm as already observed with the adoption of other technologies in the medical feld. We do not ordinarily use pleural manometry [40].
Thoracenteses are routinely performed on ambulatory patients under local anesthesia. From our standpoint of view, the use of moderate/deep sedation or general anesthesia should be limited, as feedback provided by the patient during the procedure is fundamental in knowing when to stop drainage. Fluid drainage could be accomplished either by gravity, manual aspiration using a syringe, or active aspiration with vacuum bottles or wall suction [40]. The use of aspiration decreases the procedure time when compared to gravity drainage [41].
The procedure is safe and there are no absolute contraindications other than patient’s refusal and absence of PF. It has a low incidence of complications including pneumothorax requiring intervention (0.3%), re-expansion pulmonary edema (0.1%), and serious bleeding events requiring additional intervention (0.05%) [40, 42]. We regularly perform the procedure on patients with platelet counts as low as 30 K/ μL. In patients with platelets below 30 K/μL and even those who are refractory to platelet transfusions, thoracentesis may be consider on a case- to-case basis with transfusion of platelets prior to or during the procedure [29]. In patients with coagulopathy or treated with anticoagulant/antiplatelet therapy, it is desirable to perform the thoracentesis once the abnormalities are corrected or the medications are stopped. However,
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
33 Management of Malignant Pleural Efusions |
591 |
|
|
a
b
Fig. 33.5 (a) Management of malignant pleural effusions on patients with lung re-expansion after thoracentesis. (Copyright 2021. The University of Texas MD Anderson Cancer Center (with permission)). (b)
Management of malignant pleural effusions on patients without lung re-expansion after thoracentesis. (Copyright 2021. The University of Texas MD Anderson Cancer Center (with permission))
592 |
C. A. Jiménez and V. R. Shannon |
|
|
a |
b |
c |
d |
e
Fig. 33.6 (a) 52-year-old woman with adenocarcinoma of the lung previously treated with chemotherapy and radiation. Posterioanterior chest view with complete opacifcation of the right hemithorax and ipsilateral mediastinal shift. (b) CT chest with contrast. Axial cut just below the main carina showing a right main stem obstruction, atelectasis and pleural effusion. (c) Bronchoscopy
image of the right main stem bronchus. The proximal right upper bronchus is completely obstructed. The proximal bronchus intermedius is partially obstructed. (d) Bronchoscopy image of the distal bronchus intermedius with a complete obstruction. (e) Anteroposterior chest view after draining 1250 cc of pleural uid
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
33 Management of Malignant Pleural Efusions |
593 |
|
|
when an urgent thoracentesis is needed or anticoagulation/antiplatelet therapy cannot be discontinued, the incidence of serious bleeding events is still low and the procedure should be offered [42, 43].
After a therapeutic thoracentesis, lung re- expansion, time to PF re-accumulation, performance status, life expectancy, and patient preference guide our subsequent recommendations.
Approximately 97% of MPEs will recur within 1 month after drainage, with most uid re-accumulations occurring within 1–3 days [25]. Patients with sizable pleural effusions on diagnostic imaging, larger volumes of PF drained, and higher lactate dehydrogenase (LDH) levels on PF are at an increase hazard of having a pleural effusion recurrence that requires a pleural intervention. On the other hand, those patients without malignant cells on PF cytology have a decreased hazard of PF recurrence [44].
Anti-cancer treatment may mitigate the risk of MPE recurrence avoiding then additional pleural interventions. Those malignancies that potentially respond well to anti-tumoral treatment include breast cancer, lymphoma, small cell lung cancer, germ cell tumors, prostate, ovarian and thyroid neoplasms. An initial therapeutic thoracentesis followed by a reasonable observation period to evaluate the response to treatment is acceptable in these cases [18].
Several studies have explored the potential benefts of vascular endothelial growth factor/ epidermal growth factor receptor (VEGF/EGF) signaling inhibition on patients with MPE caused by non-small cell lung cancer [45, 46]. Although some patients with EGFR mutations could initially respond to these treatments, acquired resistance to these medications and recurrence of the MPE occur frequently [47]. These is not evidence at this time supporting the use of intrapleural agents to treat MPE.
Radiation therapy directed to the mediastinum could be effective in controlling MPE particularly in patients with lymphoma [48].
Pleural Interventions for Recurrent Symptomatic MPE
After the initial evaluation and therapeutic thoracentesis, the most important step is to refer patients to a skilled team that will effectively implement a longitudinal management of MPEs. Implementation of guideline consistent care will improve patient’s quality of life by reducing the number of procedures needed, procedure-related complications, and inpatient days [49, 50]. However, there is no consensus about an optimal guideline or best procedure to date, and in addition to the previously mentioned patient-related factors (lung re-expansion, time to PF re- accumulation, performance status, life expectancy, expected response to antitumoral therapy and patient preferences), local resources, expertise, and social conditions are also important variables to consider. In most of the situations, more than one management option may be available.
Repeated therapeutic thoracenteses are our preferred alternative for MPEs that re-accumulate slowly and for those patients with limited life expectancy (<30 days) and poor performance status. Implementation of comfort measures, including oxygen supplementation and opioids, and briefng the patient and care givers with truthful information and reasonable expectations, will help providing optimal end of life care and minimizing the number of unnecessary invasive and painful procedures that could even lead to undesirable trips to the emergency department or hospital admissions. Repeated thoracenteses are also a logical approach for patients with malignancies that are expected to respond to anti- tumoral therapy; however, frequent thoracentesis may trigger the production of local cytokines and fbrin, resulting in PF loculation, which not only complicates further thoracenteses but also limits future modes of palliation [51].
Patients with good functional status and lung expansion are suited to have either and indwelling pleural catheter (IPC), pleurodesis (using chest tube thoracostomy or thoracoscopy), or a combination of both.
594 |
C. A. Jiménez and V. R. Shannon |
|
|
An indwelling tunneled pleural catheter (IPC) is considered one of the frst-line management alternatives for patients with dyspnea related to recurrent malignant pleural effusion. IPCs are placed mainly in an outpatient setting using a Seldinger wire technique [52]. Following IPC placement, the patient and caregivers follow simple instructions to drain the uid intermittently at home. When evaluated in prospective fashion daily drainage of PF via an IPC led to a higher rate of autopleurodesis and faster time to liberation from the IPC than every other day drainage [53]. Our group encourages to continue the drainage of uid, even above 1 L, until the patient develops cough or chest discomfort. or ow stops spontaneously. The incidence of autopleurodesis up to 12 weeks after IPC placement ranges from 30–70% in various reports, with most of experts agreeing that it occurs in about 40% of the cases [53–55].
When compared with patients undergoing pleurodesis using talc slurry, patients receiving IPCs had a shorter hospital stay, without differences in quality of life, pain or dyspnea improvement for up to 6 months after the procedure [56, 57]. It is also well documented that it is safe to continue with systemic anti-tumoral treatment, as the risk of IPC-related infectious complications does not increase [58, 59]. In fact, patients receiving anti-tumoral treatment after IPC placement experienced a greater improvement in quality adjusted life days as well as those patients that were more dyspneic at baseline [60].
As is true for all medical devices, IPCs are subject to post-implantation failures and complications. Timely recognition and treatment of most of these complications might prevent hospital admissions, additional interventions, and worsening quality of life.
The safety profle of IPC is acceptable and life-threatening complications are rare. Incidence of complications varies from 8% to 20% depending on the defnitions used by investigators, and most of complications seem to occur within 1 month of IPC placement [60–65].
During IPC placement, bleeding causing hemodynamic instability, is a rare complication and its true incidence is unknown, but likely less
than 1 in 1000 IPC placements. Signifcant bleeding occurs in 0.4% of the cases. Bleeding during IPC placement is generally mild and subsides with application of local pressure. Signifcant bleeding can be prevented identifying patients with risk factors before procedure, including thrombocytopenia (especially if platelet count is less than 30 K), use of anticoagulant or antiplatelet agents, and coagulation disorders (including severe liver and renal impairment). It is our practice to stop antiplatelet agents, except for aspirin that is usually continued, at least 5 days prior to intervention, re-starting them the next day after catheter insertion. Platelet counts are maintained at 30 K or higher during catheter placement and for 48 h after procedure. Anticoagulation is stopped, waiting enough time to allow for a normal coagulation function. Anticoagulants will be also re-started next day after IPC placement [29, 66]. Bleeding during chronic PF (PF) drainage using IPC is also unusual and it seldom requires additional intervention. It might be caused by local trauma on the pleura or malignant pleural implants during the evacuation maneuver using negative pressure. In the rare event a hemothorax occurs, IPC can successfully be used to drain it, while establishing all needed supportive management.
The incidence of pneumothorax during IPC placement is 5–10%. Most of the pneumothoraces are identifed immediately after IPC placement and could be the result of a non-expandable lung or room air entering the pleural cavity during IPC placement. Additional interventions are rarely needed, and patients are instructed to start drainage the following day. If there is concern a pneumothorax can worsen and/or cause deterioration of patient’s respiratory function, overnight hospital observation is warranted and repeat chest x-rays at 4 and 12 h after intervention are recommended. Pneumothorax caused by local damage of the visceral pleural during chronic PF drainage using negative pressure is rare, and generally does not require additional intervention, but only continuation of regular PF drainage. IPC can be connected preferably to a close chest tube drainage system to relief a symptomatic or tension pneumothorax and to evaluate the air leak in the subse-
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
33 Management of Malignant Pleural Efusions |
595 |
|
|
quent days. Application of suction to a close drainage system is in general discouraged unless symptoms are not relief, since it could perpetuate the air leak. Additional interventions (chest tubes, placement of intrabronchial valves or thoracotomy) are very seldom needed.
Kinked IPC is reported in 0.4% of the cases. Attention to proper catheter insertion technique is the only way to avoid this problem. It generally occurs when IPC is not properly sitting on the tunnel tract or if a suture is accidentally placed around the catheter.
Catheter-related infections are the most common complication after IPC placement and most of the studies report an incidence below 10%. Our group divides this complication in exit site infection, tunnel infection and pleural space infection. Patients with exit site infection present without fever or symptoms of systemic infection. Purulent secretion at the catheter exit site can be observed, and samples should be obtained for gram stain and culture if that is the case. Empiric oral antibiotic treatment (10 days) for methicillin- resistant Staphylococcus aureus (MRSA) should be started and adjusted according to clinical response and culture results. Patients are closely followed up during the subsequent 2 weeks. Progression of infection is unusual, and removal of IPC is rarely needed.
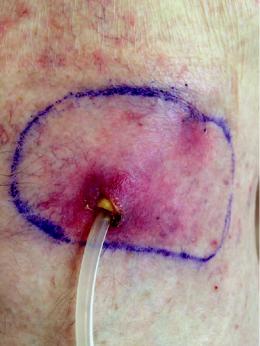
Patients with tunnel infection have erythema, tenderness and induration overlying the tunnel tract or extending greater than 2 cm from the IPC exit site (Fig. 33.7). Removal of IPC after drainage of PF is recommended in addition to empiric oral antibiotic treatment. In this situation, the removal of the IPC is fundamental to allow for proper drainage of the infection that is impeded by the catheter’s polyester cuff.
Patients with empyema might present with fever, rigors or other clinical evidence of a systemic in ammatory response. Drained PF might be purulent, but on occasions it is clear, and a diagnostic thoracentesis is recommended to prove infection of the pleural space in these situations. PF drained using the IPC should not be submitted for microbiology studies, since false positive results are common due to intraluminal catheter bacterial colonization. Drainage of uid
Fig. 33.7 Tunnel infection. Skin erythema and induration of more than 2 cm from the indwelling pleural catheter exit site, also affecting the tunnel track. Culture of exit site secretion grew S. aureus
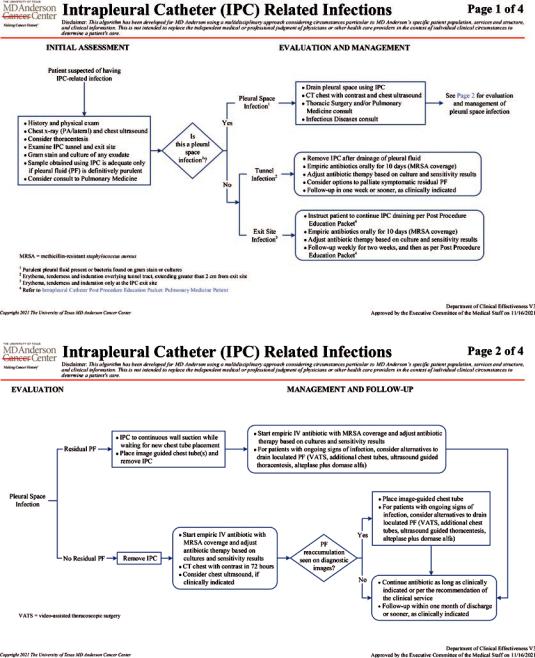
is paramount, and it can be accomplished using the IPC. Evaluation of residual uid with CT chest and thoracic ultrasound is needed, and drainage of residual PF should be accomplished either with chest tubes or thoracentesis. Thoracoscopy or thoracotomy are seldom required, and risk and benefts of these interventions should be carefully considered in patients with advanced malignant disease. In patients with empyemata and malignant pleural disease, the use of intrapleural fbrinolytic agents with or without mucolytic agents should be considered with caution, due to the increased risk of pleural hemorrhage. Empiric intravenous antibiotics to cover MRSA should be started and modifed according to culture results and clinical response. Antibiotic treatment needs to continue for 2–4 weeks following defervescence. IPC can often be removed after completing antibiotic treatment, and pleurodesis is achieved frequently. Figure 33.8a, b display our algorithm for management of IPC-related infections [67].
596 |
C. A. Jiménez and V. R. Shannon |
|
|
a
b
Fig. 33.8 (a) Management of intrapleural catheter related infections. (Copyright 2021. The University of Texas MD Anderson Cancer Center (with permission)).
(b) Management of intrapleural catheter related infections. (Copyright 2021. The University of Texas MD Anderson Cancer Center (with permission))
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
33 Management of Malignant Pleural Efusions |
597 |
|
|
Mechanical complications including obstruction or clogging of IPC have been reported in 3–9.1% of patients. It is important to correctly identify patients with this complication. Patients presenting with sudden reduction of PF drainage, having signifcant residual uid identifed by ultrasound or computed tomography on areas expected to be drained by a properly placed IPC, are very likely to have an obstructed catheter. We recommend to initially ush the IPC with 20 cc of saline solution. If drainage of PF following this maneuver remains below 150 cc, then we proceed with instillation of 4 mg of tissue plasminogen activator (tPA) diluted on 20 cc of saline solution with a dwell time of 1 h. A second 4 mg tPA dose with a dwell time of 12–24 h is used if PF drainage after the frst dose remains below 150 cc. Chest x-ray PA and lateral is requested at any time more than a 150 cc of PF are obtained, to evaluate and document successful removal of residual PF. If drainage of PF remains below 150 cc after a second tPA dose, treatment is considered a failure, and additional procedures to remove PF should be considered to alleviate patient’s dyspnea. Restoration of IPC ow with tPA is reported in about 80% of patients, with a re-occlusion incidence of 30% [68].
Leakage around the IPC is also rare (less than 2% incidence) occurring most often immediately after insertion and persisting for a few days. Increasing frequency of drainage using IPC generally resolves the problem. Leakage presenting several days after IPC placement should prompt an evaluation for a catheter-related infection.
Dislodged catheter occurs in up to 6% of patients after catheter insertion. Making small exit site incisions (0.5 cm), intentionally forcing the catheter polyester cuff into the tunnel and leaving an anchoring suture for 2 weeks, are our recommendations to prevent this complication. Patients presenting with dislodge catheters after fbrous adhesions have formed around the polyester cuff should prompt an evaluation for a catheter-related infection. All dislodge IPCs should be removed. If clinically indicated, a new IPC can be placed in a different location.
Some degree of pain related to catheter insertion is expected during the frst 48 h after IPC placement. If needed, acetaminophen or nonsteroidal anti-in ammatory drugs (NSAIDs) can be prescribed to alleviate discomfort. Also, some mild-to-moderate pleurisy is expected at the end of the drainage procedure. Its cause is not well established but might be related to shearing forces over the parietal pleura caused by PF drainage. Pleurisy improves rapidly after drainage is stopped, lasting less than 30 min. On rare occasion, pleuritic pain might be intense. Premedication 20–30 min before PF drainage with acetaminophen, NSAID, or oral narcotics is recommended in these situations. Additional maneuvers that could help decreasing pleurisy include pinching or kinking the tubing system to decrease PF ow and/or stopping drainage after reaching certain amount of uid before pain occurs. Persistent pain caused by nerve or periosteum damage and requiring removal of IPC has been reported in 0.4% of patients.
Tumor seeding of insertion tract might occur with any tumor type, but over 90% of the cases are patients with mesothelioma. The reported incidence of this complication is as low as 0.4% but was 10% in one case series. This complication develops 2 months or more after IPC insertion. Some groups advocated for prophylactic radiation therapy within 2 weeks after procedure, but there is not enough evidence to support this approach. Once catheter tract metastases developed, palliative radiation is safe and effective controlling pain and local disease progression.
Dedicated outpatient clinics to follow up patients after IPC insertion might help to promptly identify and treat complications related to catheter placement and use. Additionally, systematic data collection from these encounters will help implementing institutional quality assessment/quality improvement programs to improve patient care. Our group follows up patients 2 weeks after IPC insertion (suture removal is done at this time), and then every month for as long as the catheter is in place. Patients are evaluated clinically and radiographi-
598 |
C. A. Jiménez and V. R. Shannon |
|
|
cally with a posteroanterior and lateral chest x-ray. Patients are also encouraged to contact our clinic if they have questions or concerns related to the IPC. Clear guidelines are needed to know when IPC can be removed due to pleurodesis to avoid unnecessary additional days with IPC in place. Once daily catheter drainage is 150 cc or less for 3 consecutive days, our patients are instructed to drain PF every other day. While draining every other day, if PF is ≤150 cc in three consecutive occasions, patients are instructed to contact our clinic for evaluation of IPC removal.
Finally, placement of IPC using maximal sterile barrier precautions, on a standardized location, and with the assistance of ancillary personnel familiarized with catheter placement techniques, might decrease the incidence of catheter-related infections [65, 66].
Traditionally, pleurodesis has been the most widely used method to control recurrent MPE, and palliation might be obtained in a shorter time without the need for daily drainage of uid as it occurs with IPCs. In general, the availability of sclerosing agents and local expertise will determine what will be offer to patients. The results from available literature suggest that chemical pleurodesis using large particle, sterilized asbestos-free talc is safe, effcacious, and cost- effective [69–72]. Both, thoracoscopic talc insuf-ation and talc slurry via chest tube seem to be equally effective as a method of administration, with reported success rates of >70%, without signifcant differences in the rate of overall complications [73, 74]. The administration of talc slurry seems to be more effective using a large-bore chest tube (24 French) rather than a small-bore one (12 French), while large-bore chest tube might cause more discomfort to patients [70, 75].
Multiple complications are reported after talc pleurodesis including wound infection, empyema, persistent air leaks, pneumonia, pulmonary embolism, and acute respiratory failure [74]. However, the use of calibrated French large- particle talk has reduced the majority of the most
serious complications observed. Fever and pain are the most common post-procedure complications, but their actual incidence is unknown. Pain occurs almost invariably, and its management should be planned before the procedure is started. As expected, local practices and patient preferences affect the strategy selected to control pain, and more high-quality evidencebased information is required to properly address this aspect of pleurodesis, as there is a global desire to reduce the use of opioids. Results from a one randomized study suggest that when compared to opioids, using nonsteroidal anti- in ammatory drugs (NSAIDs) to control pain after talc slurry required more frequent rescue analgesia. However, the anti-in ammatory effect of the NSAIDs did not seem to affect pleurodesis rates [75]. New strategies using regional anesthetic blocks or epidural catheters for pain control, coupled with shorter duration of chest tube drainage and involvement of specialized pain control services, could result in higher patient satisfaction and less use of opioids [76].
Table 33.1 compares the advantages and disadvantages of IPCs and talc pleurodesis.
Inpatient and outpatient strategies combining talc pleurodesis with IPCs have been also studied, aiming at reducing the number of inpatient days and days with IPC in placed.
One approach used thoracoscopic talc insuf-ation with a chest tube placed using the thoracoscopy port and a separate IPC. Although the quality of the evidence describing this technique is not ideal, it suggests that there is a reduction in inpatient days without affecting the pleurodesis rates or increasing the number of complications [77–79].
Outpatient administration of talc slurry using a previously placed IPC has also been employed in the management of recurrent pleural effusions. A randomized trial evaluating this strategy reported a higher rate of pleurodesis when compared with standard IPC drainage (43% vs. 23% at 35 days). However, the results need to be inter-
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
33 Management of Malignant Pleural Efusions |
599 |
|
|
Table 33.1 Compared advantages and disadvantages of IPC and talc pleurodesis
|
|
Talc |
|
IPC |
pleurodesis |
Symptom palliation |
Yes |
Yes |
Pleurodesis |
≈35–45% |
≈70% |
Does palliation |
No |
Yes |
depend on pleurodesis |
|
|
success? |
|
|
Outpatient procedure |
Yes |
No |
Interruption of |
No |
Yes |
anti-cancer treatment |
|
(1–3 weeks) |
|
|
|
Lung expansion |
No |
Yes |
required |
|
|
Performance status |
Any (better |
<2 |
|
<2) |
|
Life expectancy |
Any (better |
>30 days |
|
>30 days) |
(better |
|
|
>90 days) |
Pleural uid drainage |
Yes, daily or |
No |
after intervention? |
3–4 times a |
|
|
week |
|
Social support/care |
Likely |
Unlikely |
giver needed after |
|
|
intervention? |
|
|
|
|
|
Incidence of infection |
≈5% |
≈1% |
Overall morbidity |
Low |
Low |
|
|
|
Mortality |
Low |
Low |
Percentage of patients |
≈80–90% |
≈20–30% |
with MPE that could |
|
|
be eligible for the |
|
|
intervention |
|
|
preted cautiously, considering that a large number of patients enrolled were excluded from the trial and nearly 10% of the patients assigned to a treatment arm were not included in the fnal analysis [80].
Patients with suspected non-expandable lung require further assessment to exclude the possibility of an airway obstruction amenable to endobronchial intervention before considering a pleural intervention.
An additional advantage of IPCs in the management of MPE is that their placement is appropriate whether there is an expandable lung, an important factor to ponder since nonexpendable lung might be highly prevalent (50%) among patients with MPEs [81]. Attempts at pleurodesis using any sclerosing agent or surgical technique in this group of patients is in general discourage as it is futile and it can cause unnecessary harm. Therefore, the best alternative for patients with MPE and nonexpendable lung is an IPC.
IPCs are also a suitable alternative on those patients with pleural effusions and endobronchial obstructions that are not candidates for endobronchial interventions, patients with loculated effusions and after chemical or surgical pleurodesis failures.
The frequency of pleural uid drainage using IPCs on patients with nonexpendable lung can be adjusted according to patient’s needs and symptoms. Patient with ex-vacuo hydropneumothoraces without radiographic improvement after 1 or 2 weeks of daily drainage might prefer to drain pleural uid only every several days to relieve symptoms. On the other hand, some patients might beneft from aggressive daily drainage of pleural uid and air, as some of them might have autopleurodesis (Fig. 33.9a–c). The incidence of autopleurodesis in these situations is unknown.
