
- •Contents
- •Preface to the Third Edition
- •About the Authors
- •How to Use Herbal Medicines
- •Introduction
- •General References
- •Agnus Castus
- •Agrimony
- •Alfalfa
- •Aloe Vera
- •Aloes
- •Angelica
- •Aniseed
- •Apricot
- •Arnica
- •Artichoke
- •Asafoetida
- •Avens
- •Bayberry
- •Bilberry
- •Bloodroot
- •Blue Flag
- •Bogbean
- •Boldo
- •Boneset
- •Borage
- •Broom
- •Buchu
- •Burdock
- •Burnet
- •Butterbur
- •Calamus
- •Calendula
- •Capsicum
- •Cascara
- •Cassia
- •Cat’s Claw
- •Celandine, Greater
- •Celery
- •Centaury
- •Cereus
- •Chamomile, German
- •Chamomile, Roman
- •Chaparral
- •Cinnamon
- •Clivers
- •Clove
- •Cohosh, Black
- •Cohosh, Blue
- •Cola
- •Coltsfoot
- •Comfrey
- •Corn Silk
- •Couchgrass
- •Cowslip
- •Cranberry
- •Damiana
- •Dandelion
- •Devil’s Claw
- •Drosera
- •Echinacea
- •Elder
- •Elecampane
- •Ephedra
- •Eucalyptus
- •Euphorbia
- •Evening Primrose
- •Eyebright
- •False Unicorn
- •Fenugreek
- •Feverfew
- •Figwort
- •Frangula
- •Fucus
- •Fumitory
- •Garlic
- •Gentian
- •Ginger
- •Ginkgo
- •Ginseng, Eleutherococcus
- •Ginseng, Panax
- •Golden Seal
- •Gravel Root
- •Ground Ivy
- •Guaiacum
- •Hawthorn
- •Holy Thistle
- •Hops
- •Horehound, Black
- •Horehound, White
- •Horse-chestnut
- •Horseradish
- •Hydrangea
- •Hydrocotyle
- •Ispaghula
- •Jamaica Dogwood
- •Java Tea
- •Juniper
- •Kava
- •Lady’s Slipper
- •Lemon Verbena
- •Liferoot
- •Lime Flower
- •Liquorice
- •Lobelia
- •Marshmallow
- •Meadowsweet
- •Melissa
- •Milk Thistle
- •Mistletoe
- •Motherwort
- •Myrrh
- •Nettle
- •Parsley
- •Parsley Piert
- •Passionflower
- •Pennyroyal
- •Pilewort
- •Plantain
- •Pleurisy Root
- •Pokeroot
- •Poplar
- •Prickly Ash, Northern
- •Prickly Ash, Southern
- •Pulsatilla
- •Quassia
- •Queen’s Delight
- •Raspberry
- •Red Clover
- •Rhodiola
- •Rhubarb
- •Rosemary
- •Sage
- •Sarsaparilla
- •Sassafras
- •Saw Palmetto
- •Scullcap
- •Senega
- •Senna
- •Shepherd’s Purse
- •Skunk Cabbage
- •Slippery Elm
- •Squill
- •St John’s Wort
- •Stone Root
- •Tansy
- •Thyme
- •Uva-Ursi
- •Valerian
- •Vervain
- •Wild Carrot
- •Wild Lettuce
- •Willow
- •Witch Hazel
- •Yarrow
- •Yellow Dock
- •Yucca
- •1 Potential Drug–Herb Interactions
- •4 Preparations Directory
- •5 Suppliers Directory
- •Index
Witch Hazel
Summary and Pharmaceutical Comment
Witch hazel is characterised by its tannin constituents and astringent properties. The documented herbal uses are related to these astringent properties. There is limited evidence from clinical studies to indicate that witch hazel used topically is effective in the treatment of haemorrhoids, but its use in the treatment of eczema and dermatitis is more controversial. There are no known problems associated with the use of topical preparations of witch hazel during pregnancy and lactation.
Species (Family)
Hamamelis virginiana L. (Hamamelidaceae)
Synonym(s)
Hamamelis, Virginian Witch Hazel, Witchazel
Part(s) Used
Bark, leaf
Pharmacopoeial and Other Monographs
BHP 1996(G9)
BP 2007(G84)
Complete German Commission E(G3)
ESCOP 2003(G76)
Martindale 35th edition(G85)
Ph Eur 2007(G81)
USP29/NF24(G86)
Legal Category (Licensed Products)
GSL(G37)
Constituents
The following is compiled from several sources, including Reference 1 and General References G2 and G52.
Flavonoids (leaf) Flavonols (e.g. kaempferol, quercetin) and their glycosides including astragalin, quercitrin, afzelin and myricitrin.
Tannins, catechins Pharmacopoeial standard, not less than 3%.(G81, G84) Hamamelitannin (hydrolysable), lesser amounts of condensed tannins (bark). (þ)-catechin, (þ)-gallocatechin, ( )- epicatechingallate, ( )-epigallocatechingallate, proanthocyanidin oligomers of cyanidin and delphinidin type.
Volatile oils About 0.5%. Hexen-2-ol, hexenol, a- and b-ionones, eugenol, safrole and sesquiterpenes.
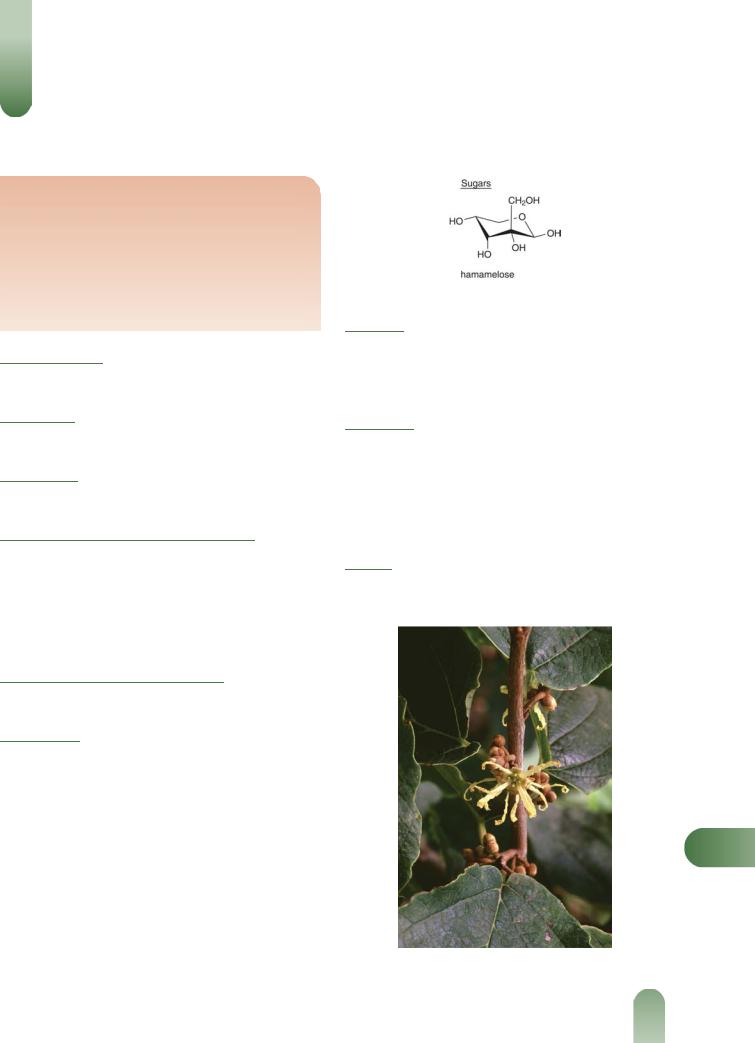
Other constituents Fixed oil (about 0.6%), resin (hamamelin, hamamamelitannin), wax, saponins, choline, free gallic acid and free hamamelose.
Figure 1 Selected constituents of witch hazel.
Food Use
Witch hazel is listed by the Council of Europe as a natural source of food flavouring (category N3). This category indicates that there is insufficient information available for an adequate assessment of potential toxicity.(G16)
Herbal Use
Witch hazel is stated to possess astringent, antihaemorrhagic and anti-inflammatory properties. Traditionally, it has been used for diarrhoea, mucous colitis, haemorrhoids, haematemesis, haemoptysis, and externally for external haemorrhoids, bruises and localised inflamed swellings. The German Commission E approved use for minor skin injuries, local inflammation of skin and mucous membranes, haemorrhoids and varicose veins.(G3)
Dosage
Dosages for oral (unless otherwise stated) administration (adults) for traditional uses recommended in older and contemporary standard herbal and pharmaceutical reference texts are given
W
Figure 2 Witch hazel (Hamamelis virginiana).
601

602 Witch Hazel
Figure 3 Witch hazel – dried drug substance (bark).
below. While doses for oral administration are given in older literature, modern use of witch hazel is by topical application.
Dried leaves 2 g as an infusion three times daily.(G6)
Hamamelis Liquid Extract (BPC 1973) 2–4 mL (1 : 1 in 45% alcohol) three times daily.(G6)
Hamamelis Water (BPC 1973) for local application, undiluted or 1 : 3 dilution for external use.(G3)
Decoction 5–10 g in 250 mL water for compresses.(G3)
Pharmacological Actions
In vitro and animal studies
Witch hazel is known to possess astringent and haemostatic properties, which have been attributed to the tannin constituents. Vasoconstriction was reduced in the hindquarters of rabbits when arteries were perfused with aqueous or ethanolic extracts of witch hazel leaf. A 70% ethanolic extract of leaf (1 : 5, 200 mg/kg, administered orally) significantly inhibited the chronic phase of carrageenan-induced rat paw oedema over a period of 19 days, compared with control (p < 0.05).(2) An aqueous ethanolic extract of witch hazel bark yielded a fraction rich in polymeric proanthocyanins after ultracentrifugation.(3) This fraction was significantly active against herpes simplex virus type 1 (HSV-1). It also showed radical scavenging properties, inhibited b-glucosidase and human leukocyte elastase activity, and was active in the croton oil ear oedema test in mice. In other studies, 3-O-galloyl- epicatechin-(4b,8)-catechin, a catechin oligomer and hamamelitannin isolated from witch hazel bark had IC50 values of 6.6, 8.8 and 1.0 mmol/L, respectively, for inhibition of 5-lipoxygenase.(4) The oligomer was active in the microsomal lyso-PAF:acetyl-CoA- acetyltransferase inhibition assay, with an IC50 value of 9.4 mmol/ L, whereas hamamelitannin was inactive.(4)
WClinical studies
Robust clinical research assessing the effects of witch hazel is limited and rigorous randomised clinical trials are required. A double-blind, controlled trial involving 90 patients with haemorrhoids compared the effects of witch hazel bark salve with those
of other salves. Witch hazel was reported to be superior in relief of symptoms.(G50)
In a study involving 30 volunteers who received topical applications of a hydroglycolic extract of witch hazel leaf, skin
temperature was significantly reduced, compared with baseline
values. This was interpreted as a possible vasoconstrictor effect of witch hazel.(G52) The effects of an after-sun lotion containing 10%
hamamelis distillate were assessed in 30 healthy volunteers using a modified UV-B erythema test for inflammation.(5) It was reported that erythema suppression ranged from 20% at seven hours to 27% at 48 hours.
In a two-week, randomised, double-blind trial, 72 patients with moderately severe eczema were treated with either a hamamelis distillate cream (5.35 g distillate with 0.64 g ketone/100 g), hydrocortisone cream 0.5%, or drug-free cream.(6) All three treatments significantly reduced itching, erythema and scaling after one week. Hydrocortisone cream was more effective than hamamelis cream.
Several clinical studies of witch hazel in the treatment of eczema have been reviewed.(G50) A double-blind, placebo-controlled trial of witch hazel salve (25% water distillate from leaf) involving 80 patients with toxic and degenerative eczema and 31 patients with endogenous eczema found that atopic dermatitis responded to the treatment to a greater extent than to placebo, but that there was no significant effect on primary irritant contact dermatitis. An uncontrolled study involving 22 patients with atopic eczema who were treated with witch hazel (4 g leaf provided 25 mL distillate/ 100 g salve) applied to affected arms over a three-week period reported improvements in symptoms, compared with baseline values.(G50) Uncontrolled studies provide preliminary data only and the observed effects cannot be attributed definitively to witch hazel. An uncontrolled study involving 37 patients treated with a witch hazel leaf cream twice daily for two weeks reported improvements in eczema and neurodermatitis.
Side-effects, Toxicity
The volatile oil contains safrole, a known carcinogen (see Sassafras), but in amounts too small to cause concern. Stomach irritation may occur in susceptible patients after oral treatment. Four of 1032 patients tested reacted to an ointment containing
25% witch hazel extract, but two of these patients were sensitive to wool fat in the ointment base.(G50)
Contra-indications, Warnings
None documented for witch hazel. In view of the tannin constituents, excessive ingestion of witch hazel is not recommended.
Pregnancy and lactation In view of the lack of information on the safety of witch hazel preparations administered orally, their use during pregnancy and lactation should be avoided. There are no known problems associated with topical use of witch hazel during pregnancy and lactation.
Preparations
Proprietary single-ingredient preparations
Australia: Optrex Original; Witch Doctor. Austria: Hametum. Canada: Optrex. Chile: Sperti Preparacion H Clear Gel. France: Optrex. Germany: Hamasana; Hametum; Posterine; Venoplant top. Italy: Acqua Virginiana; Derminiol; Optrex.
Malaysia: Optrex. Mexico: Tia Puppy. New Zealand: Optrex.
Singapore: Optrex. Spain: Derminiol; Hametol; Hemo Derminiol; Optrex. Switzerland: Mavena Anal-Gen; Optrex. Thai-
land: Optrex. UK: Optrex; Preparation H Clear Gel; Witch Doctor; Witch Sunsore. USA: A-E-R; Neutrogena Drying.
Proprietary multi-ingredient preparations
Argentina: Domuderm; Ecnagel; Esculeol P; Lavandula Oligoplex; Manzan; Venoful; VNS 45. Australia: Anusol; Bioglan Cirflo; Gentlees; Hemocane; Optrex; Proflo. Austria: Arnicet; Inotyol; Inotyol; Mirfulan; Sulgan 99; Sulgan 99; Tampositorien mit Belladonna. Belgium: Rectovasol. Brazil: Bromidrastina; Hemodotti; Hemorroidex; Malvatricin Natural Organic; Manolio; Mirorroidin; Proctosan; Supositorio Hamamelis Composto; Varizol; Varizol; Visionom. Canada: Onrectal; Penaten; Preparation H Cooling Gel; Tucks. Chile: Proctoplex. Czech Republic: Aviril H. France: Aphloine P; Climaxol; Ekseme; Evarose; Fluon; HEC; Histo-Fluine P; Jouvence de l'Abbe Soury; Jouvence de l'Abbe Soury; Jouvence de l'Abbe Soury; Keracnyl eau nettoyante; Mediflor Tisane Circulation du Sang No 12; Ophtalmine; Pastilles Monleon; Phlebosedol; Phytomelis; Veinostase. Germany: Aescusan; Chlorophyllin Salbe "Schuh"; Eulatin NN; Leukona-Wundsalbe; Mirfulan; Sagittaproct; Sanaderm; Trauma-cyl; Varicylum-S; Weleda Hamorrhoidalzapfchen. Israel: Aforinol; Derma Care; Inotyol. Italy: Centella Complex; Centella Complex; Centeril H; Centeril H; Decon Ovuli; Dermilia Flebozin; Dermitina; Dermoprolyn; Eulux; Ginoxil Ecoschiuma; Intim; Iridil; Lycia Luminique; Nevril; Proctopure; Sacnel; Salviette H; Steril Zeta; Varicogel; Venactive; Venoplus. Mexico: Almodin; Prespir; Supranettes Naturalag. New Zealand: Lacto Calamine; Optrex
Witch Hazel |
603 |
Red-Eye Relief. Portugal: Hemofissural. South Africa: Lotio Pruni Comp cum Cupro; Stibium Comp. Singapore: Stop-Itch Plus. Spain: Banoftal; Ojosbel; Roidhemo; Ruscimel; Solucion Schoum; Venofit. Switzerland: Collypan; Euproctol N; Haemocortin; Haemolan; HEC; Mavena Proctal-Gen; Oculosan; Optrex compresses; Tendro. Thailand: Opplin. UK: Adiantine; Eye Dew; Heemex; Lacto Calamine; Lacto Calamine; Modern Herbals Pile; Optrex Red Eyes; Swarm; Tea Tree & Witch Hazel Cream; Varicose Ointment; Vital Eyes. USA: Clearasil Double Clear; Preparation H Cooling Gel; Succus Cineraria Maritima; Tucks. Venezuela: Biomicovo; Camolyn; Supranettes.
References
1 Zeylstra H. Hamamelis virginiana. Br J Phytother 1998; 5: 23–28.
2Duwiejua M et al. Anti-inflammatory activity of Polygonum bistorta,
Guaiacum officinale and Hamamelis virginiana in rats. J Pharm Pharmacol 1994; 46: 286–290.
3Erdelmeier CAJ et al. Antiviral and antiphlogistic activities of
Hamamelis virginiana. Planta Med 1996; 62: 241–245.
4Hartisch C et al. Dual inhibitory activities of tannins from Hamamelis virginiana and related polyphenols on 5-lipoxygenase and lyso-PAF: acetyl-CoA acetyltransferase. Planta Med 1997; 63: 106–110.
5 Hughes-Formella BJ et al. Anti-inflammatory effect of hamamelis lotion in a UVB erythema test. Dermatology 1998; 196: 316–322.
6Korting HC et al. Comparative efficacy of hamamelis distillate and hydrocortisone cream in atopic eczema. Eur J Clin Pharmacol 1995; 48: 461–465.
W
Yarrow
Summary and Pharmaceutical Comment
The chemistry of yarrow is well-documented although there has been some disagreement over the major component in the volatile oil. Various pharmacological actions have been reported in animal studies which support many of the reputed herbal uses, although robust clinical research assessing the efficacy and safety of yarrow is limited. Yarrow is considered to be relatively non-toxic although allergic reactions in susceptible individuals have been documented. The volatile oil is contra-indicated in pregnancy and in view of the lack of
safety information, use of yarrow should be avoided during lactation.(G58)
Species (Family)
Achillea millefolium L. (Asteraceae/Compositae)
Synonym(s)
Milfoil, Millefolium
Part(s) Used
Flowerhead
Pharmacopoeial and Other Monographs
BHC 1992(G6)
BHP 1996(G9)
BP 2007(G84)
Complete German Commission E(G3)
Martindale 35th edition(G85)
Ph Eur 2007(G81)
Legal Category (Licensed Products)
GSL(G37)
Constituents
The following is compiled from several sources, including General References G2 and G6.
Acids Amino acids (e.g. alanine, aspartic acid, glutamic acid, histidine, leucine, lysine, proline, valine),(1, 2) fatty acids (e.g. linoleic, myristic, oleic, palmitic, stearic),(3, 4) and others including ascorbic acid,(5) caffeic acid,(6) folic acid,(5) salicylic acid and succinic acid.(1)
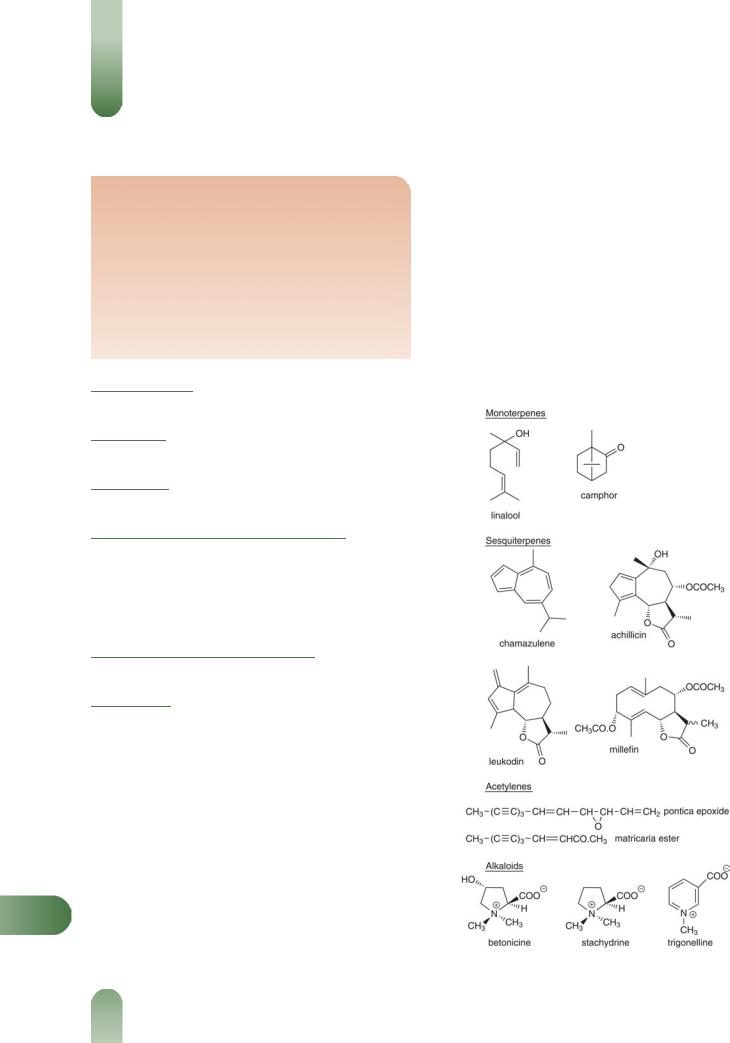
Alkaloids/bases Betonicine and stachydrine (pyrrolidine),(1, 7) trigonelline (pyridine),(1, 7) betaine and choline (bases).(1, 7) Uncharacterised alkaloids include achiceine, achilleine(8) (possible synonym for L-betonicine), which is stated to yield achilletine(7) on alkaline hydrolysis, and moscatine/moschatine,(7) stated to be an ill-defined glucoalkaloid.
YFlavonoids Predominantly flavone glycosides apigeninand luteolin-7-glycosides,(9) with lesser quantities of artemetin,
casticin, 5-hydroxy-3,6,7,4-tetramethoxyflavone and isorhamnetin.(6) Rutin (a flavonol glycoside).(5)
Tannins Condensed and hydrolysable,(3, 10) with glucose as the carbohydrate component of the latter(2)
Volatile oils Numerous identified components include borneol, bornyl acetate (trace), camphor, 1,8-cineole, eucalyptol, limonene, sabinene, terpinen-4-ol, terpineol and a-thujone (monoterpenes), caryophyllene (a sesquiterpene), achillicin, achillin, millefin and millefolide (sesquiterpene lactones), azulene and chamazulene (sesquiterpene lactonederived) and isoartemisia ketone. The relative composition of the components varies greatly between Achillea species, especially the azulene content. Azulene has been reported as the major component.(11) However, true yarrow (A. millefolium) is thought to be hexaploid and azulene-free, whereas closely related species, such as Achillea lanulosa Nutt. and Achillea collina Becker, are tetraploid and contain up to 50% azulene in their volatile oil.(5, 10, 11) It is possible that the tetraploid
Figure 1 Selected constituents of yarrow.
604

species may be supplied for A. millefolium. The azulenes are not present in the fresh herb: they are formed as artefacts during steam distillation of the oil, from unstable precursors called proazulenes (e.g. achillin and achillicin), via equally unstable azulene–carboxylic acid intermediates.(12)
Other constituents Unknown cyanogenetic compound,(13) sugars including arabinose, galactose, dextrose, dulcitol, glucose, inositol, maltose, mannitol and sucrose.(1, 2)
Food Use
Yarrow is listed by the Council of Europe as a natural source of food flavouring (herb, flowers, essential oil and other prepara-
tions: category 4, with limits on camphor, eucalyptol and thujone) (see Appendix 3).(G17) Previously, in the USA, yarrow was only
approved for use in alcoholic beverages, and the finished product had to be thujone free.(G41)
Herbal Use
Yarrow is stated to possess diaphoretic, antipyretic, hypotensive, astringent, diuretic and urinary antiseptic properties. Traditionally, it has been used for bruises, swellings, strains, fevers, common cold, essential hypertension, amenorrhoea, dysentery, diarrhoea, and specifically for thrombotic conditions with
hypertension, including cerebral and coronary thromboses.(G2, G6,
G7, G8, G64)
Dosage
Dosages for oral administration (adults) for traditional uses recommended in standard herbal reference texts are given below.
Dried herb 2–4 g as an infusion three times daily.(G6, G7)
Liquid extract 2–4 mL (1 : 1 in 25% alcohol) three times
daily.(G6, G7)
Tincture 2–4 mL (1 : 5 in 45% alcohol) three times daily.(G6, G7)
Figure 2 Yarrow (Achillea millefolium).
Yarrow 605
Figure 3 Yarrow – dried drug substance (flowerhead).
Pharmacological Actions
Some activities documented for yarrow are associated with the azulene constituents, although it is now thought that azulene is absent from true yarrow (see Constituents). It is possible that some of the documented pharmacological studies have used
Achillea species other than A. millefolium.(14)
In vitro and animal studies |
|
|
Anti-inflammatory activity has been documented for an aqueous |
|
|
extract of yarrow using mouse(15) and rat(16) paw oedema models, |
|
|
with inflammation induced by yeast(15) and various inflammatory |
|
|
substances,(16) including histamine, carrageenan and prostaglan- |
|
|
din. In mouse studies, the active fraction was reported as a series |
|
|
of protein–carbohydrate complexes. Topical anti-inflammatory |
|
|
activity in rabbits has also been documented for the aqueous |
|
|
extract.(15) In general, anti-inflammatory properties are associated |
|
|
with azulenes (see Chamomile, German). Anti-inflammatory |
|
|
activity has been described for the azulene components docu- |
|
|
mented for the volatile oil of yarrow.(5) |
|
|
A diuretic effect was also noted in mice administered an |
|
|
aqueous extract of yarrow,(15) but only at a dose more than double |
|
|
that required for an anti-inflammatory effect.(15) Terpinen-4-ol, |
|
|
the diuretic principle in juniper, has been reported as a component |
|
|
of yarrow volatile oil. |
|
|
CNS-depressant activity has been documented for the volatile |
|
|
oil: a dose of 300 mg/kg decreased the spontaneous activity of |
|
|
mice and lowered the body temperature of rats. In addition, 300– |
|
|
600 mg/kg doses inhibited pentetrazole-induced convulsions and |
|
|
prolonged sleep induced by a barbiturate preparation.(17) |
|
|
Moderate antibacterial activity has been documented for an |
|
|
ethanolic extract of the herb against Staphylococcus aureus, |
|
|
Bacillus subtilis, Mycobacterium smegmatis, Escherichia coli, |
|
|
Shigella sonnei and Shigella flexneri.(18) Antimicrobial properties |
|
|
have been documented for the sesquiterpene lactone fraction.(5) |
|
|
Achilleine 0.5 g/kg by intravenous injection has been noted to |
|
|
decrease the blood clotting time in rabbits by 32%.(8) The |
|
|
haemostatic action persisted for 45 minutes with no observable |
|
|
toxic effects. |
|
|
Antispasmodic activity on the isolated rabbit intestine has been |
|
|
documented for a flavonoid-containing fraction of yarrow.(9) |
Y |
|
Antispasmodic activity is generally associated with |
azulene |
|
constituents (see Chamomile, German). |
|
|
Antipyretic and hypotensive actions have been reported for the |
|
|
basic fraction (alkaloid/base);(G41) the sesquiterpene |
lactone |
|

606 Yarrow
fraction is stated to possess cytotoxic activities,(5) although no further details were located. Tannins are known to possess astringent activity.
Clinical studies
There is a lack of clinical research assessing the effects of yarrow and rigorous randomised controlled clinical trials are required.
Side-effects, Toxicity
Clinical data
Allergic reactions to yarrow (e.g. dermatitis) have been documented, and positive patch tests have been produced in individuals sensitised to other plants.(5, G33, G51) An instance of yarrow tea causing a generalised eruption in a sensitised individual was reported in 1929. The allergenic properties of some sesquiterpene lactones are well documented, although none of those present in yarrow are recognised sensitisers.(G51) Yarrow has been suspected of being a photosensitiser, although extracts have been reported to lack phototoxicity and to be devoid of psoralens, compounds with known photosensitising properties.(G51)
Preclinical data
Yarrow is considered to be of low toxicity. In mice LD50 values have been reported of up to 3.65 g/kg (by mouth), 3.1 g/kg (by intraperitoneal injection), and greater than or equal to 1 g/kg (by subcutaneous injection).(15, 17) In rats, an LD50 (subcutaneous injection) has been recorded as 16.86 g/kg, with corresponding LD0 and LD100 values reported as 12 and 20 g/kg, respectively.(16) By comparison, an ED25 for anti-inflammatory activity has been estimated as about 0.43 g/kg.(16)
The known toxic principle thujone has been documented as a minor component of yarrow volatile oil, although concentrations present are probably too low to represent a risk to human health.
A single report of animal poisoning has been documented for yarrow in which a calf died following the ingestion of a single plant.(5) No additional reports of animal toxicity were located.
Contra-indications, Warnings
Yarrow may cause an allergic reaction in sensitive individuals, especially those with an existing hypersensitivity to other members of the Asteraceae/ Compositae.(19)
Drug interactions None documented. However, the potential for preparations of yarrow to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should be considered. There is limited evidence from preclinical studies that achilleine, a constituent of yarrow, has anticoagulant activity, although the clinical relevance of this, if any is not clear.
Pregnancy and lactation Yarrow should not be taken during
Ymenstrual cycle,(G30) and the volatile oil contains trace amounts (0.3%) of the abortifacient principle thujone. In view of the lack
of safety information use of yarrow should be avoided during lactation.pregnancy. It is reputed to be an abortifacient and to affect the
Preparations
Proprietary single-ingredient preparations
Czech Republic: Gallentee; Nat Rebricku; Rebrickovy Caj, Rebrickova Nat. Mexico: Blancaler.
Proprietary multi-ingredient preparations
Australia: Flavons. Austria: Abfuhrtee St Severin; Amersan; Gallenund Lebertee St Severin; Mariazeller; Menodoron. Canada: Original Herb Cough Drops. Czech Republic: Amersan; Cajova Smes pri Redukcni Diete; Cicaderma; Hemoral; Hertzund Kreislauftee; Perospir; Projimava; Species Urologicae Planta; Stomatosan; Ungolen; Zaludecni Cajova Smes. France: Gonaxine; Tisane Hepatique de Hoerdt. Germany: Alasenn; Amara-Tropfen; Floradix Multipretten N; Gallexier; Kamillan Plus; Sedovent; Stomachysat N; Tonsilgon. Hungary: Hemorid; Noditran. Italy: Forticrin; Lozione Same Urto; Pik Gel. Portugal: Cicaderma; Fade Cream. Russia: Liv 52 (Лив 52); Liv 52 (Лив 52); Original Grosser Bittner Balsam (Оригинальный Большой Бальзам Биттнера); Tonsilgon N (Тонзилгон Н). South Africa: Amara; Clairo; Menodoron. Spain: Jaquesor; Menstrunat; Natusor Circusil; Natusor Gastrolen; Natusor Jaquesan. Switzerland: Gastrosan; Kernosan Heidelberger Poudre; Pommade au Baume; Tisane hepatique et biliaire; Tisane pour l'estomac. UK: Catarrh-eeze; Drops of Life Tablets; Rheumatic Pain Remedy; Rutin Compound Tablets; Tabritis; Tabritis Tablets; Wellwoman.
References
1Ivanov Ch, Yankov L. Composition of Achillea millefolium. I. Preparation of the total extracts and composition of the part of the
alcoholic extracts soluble in alcohol and water. God Vissh Khimikotekhnol Inst Sofia 1967; 14: 195–222.
2 Ivanov Ch, Yankov L. Composition of Achillea millefolium. III. Composition of the parts soluble in water and insoluble in alcohol.
God Vissh Khimikotekhnol Inst Sofia 1967; 14: 223–241.
3Ivanov Ch, Yankov L. Composition of Achillea millefolium. III. Composition of the acidic, water-insoluble part of the alcoholic
extract. God Vissh Khimikotekhnol Inst Sofia 1967; 14: 61–72.
4Ivanov Ch, Yankov L. Composition of Achillea millefolium. V. Composition and structure of the components of neutral fraction insoluble in the aqueous part of the alcoholic extract. God Vissh
Khimikotekhnol Inst Sofia 1967; 14: 73–101.
5Chandler RF et al. Ethnobotany and phytochemistry of yarrow,
Achillea millefolium, Compositae. Economic Bot 1982; 36: 203–223.
6Falk AJ et al. Isolation and identification of three new flavones from
Achillea millefolium L. J Pharm Sci 1975; 64: 1838–1842.
7Zirvi KA, Ikram M. Alkaloids of some of the plants of the Compositae. Pakistan J Sci Ind Res 1975; 18: 93–101.
8Miller FM, Chow LM. Alkaloids of Achillea millefolium L. I. Isolation and characterization of Achilleine. J Am Chem Soc 1954; 76:
1353–1354.
9Hoerhammer L. Flavone concentration of medical plants with regard to their spasmolytic action. Congr Sci Farm Conf Commun 21st Pisa
1961; 578–588.
10Falk AJ et al. The constituents of the essential oil from Achillea millefolium L. Lloydia 1974; 37: 598–602.
11Haggag MY et al. Thin layer and gas-chromatographic studies on the essential oil from Achillea millefolium. Planta Med 1975; 27: 361–366.
12Sticher O. Plant mono-, diand sesquiterpenoids with pharmacological and therapeutical activity. In: Wagner H, Wolff P, eds. New Natural Products with Pharmacological Biological or Therapeutical Activity. Berlin: Springer Verlag, 1977: 137–176.
13Seigler DS. Plants of the Northeastern United States that produce cyanogenic compounds. Economic Bot 1976; 30: 395–407.

|
|
|
Yarrow |
607 |
14 |
Chandler F. Vindication of maritime Indian herbal remedies. J |
17 |
Kudrzycka-Bieloszabska FW, Glowniak K. Pharmacodynamic |
|
|
Ethnopharmacol 1983; 9: 323–327. |
|
properties of oleum chamomillae and oleum millefolii. Diss Pharm |
|
15 |
Goldberg AS et al. Isolation of anti-inflammatory principles from |
|
Pharmacol 1966; 18: 449–454. |
|
|
Achillea millefolium (Compositae). J Pharm Sci 1969; 58: 938–941. |
18 |
Moskalenko SA. Preliminary screening of far-Eastern ethnomedicinal |
|
16 |
Shipochliev T, Fournadjiev G. Spectrum of the antiinflammatory effect |
|
plants for antibacterial activity. J Ethnopharmacol 1986; 15: 231–259. |
|
|
of Arctostaphylos uva ursi and Achilea millefolium, L. Probl Vutr Med |
19 |
Mathias CGT et al. Plant dermatitis – patch test results (1975–78). |
|
|
1984; 12: 99–107. |
|
Note on Juniperus extract. Contact Dermatitis 1979; 5: 336. |
|
Y
