
- •Preface
- •Contents
- •1 Elements of the Nervous System
- •2 Somatosensory System
- •3 Motor System
- •4 Brainstem
- •5 Cerebellum
- •6 Diencephalon and Autonomic Nervous System
- •7 Limbic System
- •8 Basal Ganglia
- •9 Cerebrum
- •10 Coverings of the Brain and Spinal Cord; Cerebrospinal Fluid and Ventricular System
- •Further Reading
- •Index
- •Abbreviations
- •1 Elements of the Nervous System
- •Elements of the Nervous System
- •Information Flow in the Nervous System
- •Synapses
- •Neurotransmitters and Receptors
- •Functional Groups of Neurons
- •Glial Cells
- •Development of the Nervous System
- •2 Somatosensory System
- •Peripheral Nerve, Dorsal Root Ganglion, Posterior Root
- •Peripheral Regulatory Circuits
- •Central Components of the Somatosensory System
- •Posterior and Anterior Spinocerebellar Tracts
- •Posterior Columns
- •Anterior Spinothalamic Tract
- •Lateral Spinothalamic Tract
- •Other Afferent Tracts of the Spinal Cord
- •Central Processing of Somatosensory Information
- •Somatosensory Deficits due to Lesions at Specific Sites along the Somatosensory Pathways
- •3 Motor System
- •Central Components of the Motor System and Clinical Syndromes of Lesions Affecting Them
- •Motor Cortical Areas
- •Corticospinal Tract (Pyramidal Tract)
- •Corticonuclear (Corticobulbar) Tract
- •Other Central Components of the Motor System
- •Lesions of Central Motor Pathways
- •Peripheral Components of the Motor System and Clinical Syndromes of Lesions Affecting Them
- •Clinical Syndromes of Motor Unit Lesions
- •Complex Clinical Syndromes due to Lesions of Specific Components of the Nervous System
- •Spinal Cord Syndromes
- •Vascular Spinal Cord Syndromes
- •Nerve Root Syndromes (Radicular Syndromes)
- •Plexus Syndromes
- •Peripheral Nerve Syndromes
- •Syndromes of the Neuromuscular Junction and Muscle
- •4 Brainstem
- •Surface Anatomy of the Brainstem
- •Medulla
- •Pons
- •Midbrain
- •Olfactory System (CN I)
- •Visual System (CN II)
- •Eye Movements (CN III, IV, and VI)
- •Trigeminal Nerve (CN V)
- •Facial Nerve (CN VII) and Nervus Intermedius
- •Vagal System (CN IX, X, and the Cranial Portion of XI)
- •Hypoglossal Nerve (CN XII)
- •Topographical Anatomy of the Brainstem
- •Internal Structure of the Brainstem
- •5 Cerebellum
- •Surface Anatomy
- •Internal Structure
- •Cerebellar Cortex
- •Cerebellar Nuclei
- •Connections of the Cerebellum with Other Parts of the Nervous System
- •Cerebellar Function and Cerebellar Syndromes
- •Vestibulocerebellum
- •Spinocerebellum
- •Cerebrocerebellum
- •Cerebellar Tumors
- •6 Diencephalon and Autonomic Nervous System
- •Location and Components of the Diencephalon
- •Functions of the Thalamus
- •Syndromes of Thalamic Lesions
- •Thalamic Vascular Syndromes
- •Epithalamus
- •Subthalamus
- •Hypothalamic Nuclei
- •Afferent and Efferent Projections of the Hypothalamus
- •Functions of the Hypothalamus
- •Sympathetic Nervous System
- •Parasympathetic Nervous System
- •Visceral and Referred Pain
- •7 Limbic System
- •Anatomical Overview
- •Internal and External Connections
- •Microanatomy of the Hippocampal Formation
- •Amygdala
- •Functions of the Limbic System
- •Types of Memory
- •8 Basal Ganglia
- •Preliminary Remarks on Terminology
- •The Role of the Basal Ganglia in the Motor System: Phylogenetic Aspects
- •Connections of the Basal Ganglia
- •Function and Dysfunction of the Basal Ganglia
- •Clinical Syndromes of Basal Ganglia Lesions
- •9 Cerebrum
- •Development
- •Gross Anatomy and Subdivision of the Cerebrum
- •Gyri and Sulci
- •Histological Organization of the Cerebral Cortex
- •Laminar Architecture
- •Cerebral White Matter
- •Projection Fibers
- •Association Fibers
- •Commissural Fibers
- •Functional Localization in the Cerebral Cortex
- •Primary Cortical Fields
- •Association Areas
- •Frontal Lobe
- •Coverings of the Brain and Spinal Cord
- •Dura Mater
- •Arachnoid
- •Pia Mater
- •Cerebrospinal Fluid Circulation and Resorption
- •Arteries of the Anterior and Middle Cranial Fossae
- •Arteries of the Posterior Fossa
- •Collateral Circulation in the Brain
- •Dural Sinuses
- •Venous Drainage
- •Cerebral Ischemia
- •Arterial Hypoperfusion
- •Particular Cerebrovascular Syndromes
- •Impaired Venous Drainage from the Brain
- •Intracranial Hemorrhage
- •Intracerebral Hemorrhage (Nontraumatic)
- •Subarachnoid Hemorrhage
- •Subdural and Epidural Hematoma
- •Impaired Venous Drainage
- •Spinal Cord Hemorrhage and Hematoma
- •Further Reading
- •Index

6282 · 6 Diencephalon and Autonomic Nervous System
Functions of the Hypothalamus
The hypothalamus is the hierarchically uppermost regulatory organ (“head ganglion”) of the autonomic nervous system. It plays the leading role in a wide variety of regulatory circuits for vital bodily functions such as temperature, heart rate, blood pressure, respiration, and food and water intake. These regulatory functions are carried out largely independently of any conscious thought on the part of the individual, i.e., autonomically. The hypothalamus also regulates important hormone systems through the hypothalamicpituitary axis and coordinates the interaction of the endocrine and autonomic nervous systems. The elementary functions controlled by the hypothalamus will be described, briefly and individually, in this section.
Temperature Regulation
The anterior preoptic hypothalamus contains specific receptors for the maintenance of a constant internal temperature (temperature homeostasis). Physiological responses to temperature changes (vasoconstriction and shivering at low temperature, vasodilation and sweating at high temperature) are regulated by circuits in the posterior hypothalamus.
Disturbances of temperature regulation. Dysfunction of the anterior preoptic region of the hypothalamus (caused, for example, by traumatic brain injury or hemorrhage) can lead to central hyperthermia. Dysfunction of the posterior region can lead to hypothermia or poikilothermia (rapid fluctuations of body temperature by more than 2°C); the possible causative lesions here include hypothalamic tumors (craniopharyngioma, glioma), Wernicke’s encephalopathy, and hydrocephalus.
Regulation of Heart Rate and Blood Pressure
The hypothalamus influences the autonomic nervous system directly through descending pathways that will be discussed below in the section on the autonomic nervous system (p. 289).
The sympathetic nervous system is regulated by the ventromedial and posterior portions of the hypothalamus (p. 292). Stimulation of these areas induces a rise in heart rate and blood pressure, dilatation of the pupils, vasoconstriction in the capillary beds, vasodilation in the skeletal musculature, and expressions of fear or rage.
The parasympathetic nervous system (p. 295), on the other hand, is regulated by the paraventricular and anterior or lateral portions of the hypothalamus. Stimulation of these areas induces a fall in heart rate and blood pres-
Baehr, Duus' Topical Diagnosis in Neurology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.

Hypothalamus · 283 6
sure and constriction of the pupils. Stimulation of posterior parasympathetic areas increases blood flow to the bladder and diminishes blood flow to skeletal muscle.
Regulation of Water Balance
The hypothalamic osmoreceptors are located in the supraoptic and paraventricular nuclei. They are stimulated either by intracellular dehydration, with an elevated intracellular sodium concentration, or by extracellular dehydration, with an elevated concentration of angiotensin II in the hypothalamic capillary blood; stimulation leads to the secretion of ADH (antidiuretic hormone, vasopressin). Conversely, an increase of intravascular volume stimulates peripheral volume receptors, ultimately leading to the inhibition of ADH secretion.
Disturbances of water balance. If 90% or more of the neurons of the supraoptic and paraventricular nuclei are destroyed or rendered dysfunctional (e. g., by a granulomatous process, vascular lesion, trauma, or infection), then ADH is no longer secreted and diabetes insipidus results, manifested clinically by excessive thirst, polyuria, and polydipsia. The diagnosis is established by the demonstration of hypo-osmolar polyuria, i.e., the excretion of at least 3 liters of urine per day, with an osmolality between 50 and 150 mosm/l. ADH substitution is the treatment of choice. If the urine osmolality fails to rise by more than 50% after the administration of 5 IU of ADH, then the patient is suffering from renal diabetes insipidus (inadequate response of the kidney to circulating ADH), in which substitution therapy is of no help.
Many types of hypothalamic lesion impair the thirst response, and can thus cause severe hyponatremia.
The syndrome of inappropriate ADH secretion (SIADH or Schwartz-Bartter syndrome), usually caused by abnormal ectopic secretion of ADH (e. g., by bronchial carcinoma or other malignant tumors), is manifested by hypervolemia, hyponatremia ( 130 mmol/l), low serum osmolarity ( 275 mosm/ kg), and highly concentrated urine. The clinical manifestations include weight gain, weakness, nausea, and disturbances of consciousness, as well as epileptic seizures. SIADH is treated by eliminating the underlying cause, though it is often useful to treat the hypervolemia and hyponatremia symptomatically as well, by fluid restriction and correction of the sodium balance.
Baehr, Duus' Topical Diagnosis in Neurology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.

6284 · 6 Diencephalon and Autonomic Nervous System
Regulation of Nutritional Intake
Lesions of the ventromedial hypothalamic nuclei may cause severe obesity through hyperphagia and poverty of movement. More lateral lesions can cause anorexia and abnormal weight loss.
Neurosecretion and Regulation of the Endocrine System
As mentioned above, the hypophysis (pituitary gland) has two components, the anterior lobe (adenohypophysis) and the posterior lobe (neurohypophysis). The hypothalamus controls each part differently.
Hormone secretion by the posterior lobe. Secretory neurons in the supraoptic and paraventricular nuclei produce oxytocin and ADH, which are transported intra-axonally to the neurohypophysis and released there into the bloodstream (neurosecretion). The functions of ADH have been described above. Oxytocin is secreted during the last few weeks of pregnancy; it induces the contraction of uterine smooth muscle as well as the secretion of milk from the mammary glands. Somatosensory stimulation (touching the nipple) produces afferent impulses that activate the neurosecretory neurons of the hypothalamus (by way of the thalamus and the cerebral cortex). The intimate connection between this regulatory circuit and emotion is illustrated by the fact that milk production decreases significantly when the mother suffers from fear or stress.
Hormone secretion by the anterior lobe. The parvocellular secretory neurons found in periventricular areas of the hypothalamus communicate with the adenohypophysis not by axonal connections (as in the case of the neurohypophysis) but rather through a portal vascular system (see above). These parvocellular neurons secrete the “hypophysiotropic” hormones gonadotropin-releasing hormone (GnRH), thyrotropin-releasing hormone (TRH), corticotropin-releas- ing hormone (CRH), growth-hormone-releasing hormone (GHRH), and factors regulating the secretion of melanocyte-stimulating hormone (MSH), namely MIF and MRF. All of these hormones, in turn, control the release of the corresponding pituitary hormones from the adenohypophysis, once they arrive there by way of the portal vascular network (cf. Fig. 6.12 and Table 6.1). In the adenohypophysis, acidophil cells (α cells) secrete growth hormone (GH, also called somatotropic hormone or STH) and prolactin (PRL, also called luteotropic hormone or LTH). Basophil cells ( cells) secrete thyrotropin (thy- roid-stimulating hormone, TSH), corticotropin (also called adrenocorticotropic hormone or ACTH), melanocyte-stimulating hormone (MSH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH). Chromophobe cells
Baehr, Duus' Topical Diagnosis in Neurology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.

license of conditions and terms to subject Usage .reserved rights All |
Thieme 2005 © Neurology in Diagnosis Topical Duus' Baehr, |
. |
|
Somatostatin |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Oxytocin |
Hypothalamus |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
(SRIF) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vasopressin |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Neurosecretion |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pituitary |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Neurohypophysis |
gland |
|
|
|
|
|
|
|
|
Adenohypophysis |
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Kidney, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Growth, |
|
|
Lactation |
Thyroid |
Gonadal |
Adrenal |
Lipolysis |
|
|
Pig- |
|
||||||||||||
metabolism |
function |
function |
function |
|
|
|
menta- |
uterus, |
Periphery |
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
tion |
breast |
|
||
|
|
|
|
Inhibitory |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
Stimulatory |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
ACTH |
Adrenocorticotropic hormone (corticotropin) |
MRF |
Melanocyte-stimulating hormone-releasing factor |
|
|||||||||||||||||||
CRF |
Corticotropin-releasing factor |
|
|
MSH |
Melanocyte-stimulating hormone |
|
|||||||||||||||||
FSH |
Follicle-stimulating hormone |
|
|
PIF |
Prolactin-inhibiting factor (= dopamine) |
|
|||||||||||||||||
GH (STH) |
Growth hormone (somatotropic hormone) |
PRL |
Prolactin |
|
|
|
|
|
|
|
|||||||||||||
GHRH |
Growth-hormone-releasing hormone |
|
|
PRF |
Prolactin-releasing factor |
|
|
||||||||||||||||
LH |
Luteinizing hormone |
|
|
|
|
SRIF |
Somatotropin-inhibiting factor |
|
|
||||||||||||||
GnRH |
Gonadotropin-releasing hormone |
|
|
TRH |
Thyrotropin-releasing hormone |
|
|
||||||||||||||||
LPH |
Lipotropin |
|
|
|
|
TSH |
Thyroid-stimulating hormone |
|
|
||||||||||||||
MIF |
Melanocyte-stimulating hormone-inhibiting factor |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Axis Pituitary—Hypothalamic the along Regulation Endocrine 1.6 Tabelle |
Hypothalamus |
|
285 · |
|
|
6

6286 · 6 Diencephalon and Autonomic Nervous System
(γ cells) are not known to secrete any hormones, but some authors state that they play a role in ACTH synthesis.
The hormones produced by the pituitary secretory cells enter the bloodstream and induce the respective peripheral endocrine organs to secrete hormones. These peripheral hormones circulate in the blood, and their concentrations, in turn, influence the secretion of the corresponding hypothalamic and pituitary hormones, in a negative feedback loop.
Hormonal Disturbances: Disturbances of the Hypothalamic−Pituitary Axis
The endocrine function of the hypophysis can be impaired by hormone-secret- ing tumors (e. g., pituitary adenoma) or by destruction of pituitary tissue by non-hormone-secreting tumors.
Panhypopituitarism. The most severe clinical syndrome consists of loss of all functions of the hypophysis and is clinically manifested by lack of drive, decline of physical performance, loss of weight, loss of libido, bradycardia, lessened skin pigmentation, loss of axillary and pubic hair, and, sometimes, diabetes insipidus (if the neurohypophysis is involved). This syndrome may be caused by large, hormonally inactive tumors of the hypophysis, infundibulum, or hypothalamus (e. g., adenoma, metastasis, glioma, or craniopharyngioma). The treatment of choice is surgical resection and hormone substitution. Hypopituitarism may also arise in the aftermath of trauma, or as a complication of neurosurgical procedures. Sudden loss of pituitary function with subsequent adrenal failure (addisonian crisis) is a life-threatening event.
Hormone-secreting pituitary tumors. A neoplasm arising from one of the cell types of the anterior pituitary lobe causes symptoms through an excess of the corresponding hormone(s). If the tumor is large enough, the suprasellar mass effect will produce a characteristic visual field defect (usually bitemporal hemianopsia, because of compression of the optic chiasm; cf. p. 131).
Prolactinoma. Most pituitary adenomas (6070%) secrete prolactin. In female patients, the resulting excess of circulating prolactin (hyperprolactinemia) causes secondary amenorrhea through the inhibition of gonadotropin-releas- ing hormone secretion (when the serum prolactin concentration rises above 40100 ng/ml), as well as galactorrhea and, less commonly, hirsuitism. In male patients, hyperprolactinemia causes impotence, gynecomastia, and galactorrhea. Surgical resection (e. g., by the transsphenoidal route) is the treatment of choice for prolactinomas with mass effect; for smaller tumors with less severe manifestations, pharmacological treatment with a dopamine agonist such as bromocriptine can be tried. Dopamine agonists inhibit prolactin secretion.
Baehr, Duus' Topical Diagnosis in Neurology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
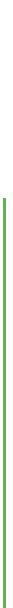
Hypothalamus · 287 |
6 |
|
|
Growth-hormone-secreting adenoma. Clinically, an excess of circulating growth hormone ( 5 ng/ml) causes acromegaly: increased growth of acral portions of the skeleton (hands, feet, head circumference), osteoporosis, hyperhidrosis, glucose intolerance, hypertension, hypertrophic cardiomyopathy, goiter, compressive neuropathies such as carpal tunnel syndrome, other types of neuropathy, proximal myopathy, sleep disturbances (hypersomnia, sleep apnea syndrome), and neuropsychiatric disturbances (depression, psychosis). The standard diagnostic test is an oral glucose tolerance test, with a characteristic overshoot in the reflex rise of growth hormone concentration. Surgical resection is the treatment of choice.
Case Presentation 2: Pituitary Tumor/Prolactinoma
This 40-year-old male office worker complained to his family physician of “peculiar” bodily changes that had been troubling him for some time. He had gained 50 kg in weight over the previous 2−3 years, and he now needed shoes two sizes larger than before. His hands also seemed to have become “rough.” He had recently had an automobile accident caused by his failure to see another car approaching from the side, and a couple of days previously he had almost run over a pedestrian for the same reason. He could no longer trust himself to drive a car, both because of these occurrences and because he was always tired and could not concentrate. He had increasing difficulty on the job. He denied suffering from headache, loss of libido, or impotence.
The physician found his weight to be 132 kg (previously 82 kg), with an unchanged height of 193 cm. His hands and feet were disproportionately large (acromegaly), finger perimetry revealed severe bitemporal hemianopsia, and there was mild gynecomastia, though no galactorrhea could be induced. Laboratory testing revealed normal values of all thyroid parameters (T3, T4, basal TSH, and TRH test) as well as of ACTH and cortisol. The testosterone level, however, was very low (50 ng/ml) and the prolactin level extremely high (590 μg/dl). TRH administration caused the prolactin level to climb still further to 2020 μg/dl.
These findings suggested a prolactin-secreting adenoma of the pituitary gland with partial hypopituitarism affecting the anterior lobe hormones, particularly the gonadotropic axis.
A plain radiograph of the head revealed massive expansion of the sella turcica with partial destruction of the dorsum sellae and the sellar floor. An MRI scan revealed a tumor measuring 5 × 5 × 4 cm (Fig. 6.13), too large to be removed through a transsphenoidal approach. A frontotemporal craniotomy was performed. Intraoperatively a firm, grayish-yellow tumor with some reddish areas was found; it was adherent to the floor of the middle cranial fossa, made contact with the terminal portion of the internal carotid artery, and compressed the optic chiasm. The histopathological finding was of a diffusely growing epithelial tumor, without lobular structure, in which the tumor cells occasionally showed a papillary organization. Immmunohistochemical study revealed an increased expression of prolactin in ca. 30−40 % of the tumor cells, while a few of them stained positive for ACTH, LH, or GH. Excessive GH secretion had presumably caused the patient’s clinically evident acromegaly. Postoperatively, he had transient diabetes insipidus requiring treatment with desmopressin acetate. Anterior pituitary lobe insufficiency persisted in his subsequent course and he was treated with hydrocortisone and thyroxine substitution.
Baehr, Duus' Topical Diagnosis in Neurology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
6 |
288 · 6 Diencephalon and Autonomic Nervous System |
|
|
a |
b |
Fig. 6.13 A large pituitary tumor (prolactinoma) in a 40-year-old man, seen in coronal (a, b) and sagittal (c) T1-weighted MR images. Images b and c were obtained after intravenous
administration of contrast material. The large intrasellar and suprasellar tumor places the optic chiasm under tension from below, stretching it (a). There is marked contrast enhancement
(b, c). The sella turcica is markedly expanded (c).
c
ACTH-secreting adenoma causes Cushing syndrome with truncal obesity, moon facies, glucose intolerance, hypertension, edema, amenorrhea, impotence, a tendency to thromboembolism, polyuria, steroid myopathy, and neuropsychiatric disturbances. The diagnosis is made endocrinologically by the demonstration of an elevated amount of cortisol in a 24-hour urine collection. Surgical resection is the treatment of choice.
Baehr, Duus' Topical Diagnosis in Neurology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.

Peripheral Autonomic Nervous System · 289 6
Peripheral Autonomic Nervous System
Fundamentals
The autonomic nervous system, working in concert with the endocrine system (see above) and various nuclei in the brainstem, regulates vital functions that are necessary for the maintenance of the internal environment (homeostasis), including respiration, circulation, metabolism, body temperature, water balance, digestion, secretion, and reproductive function. The designation “autonomic” is derived from the fact that these functions are controlled by unconscious (involuntary) mechanisms, as discussed above.
As already mentioned, the hypothalamus is the main regulatory center for the entire peripheral autonomic system. It exercises its control over many bodily functions partly through nerve impulses and partly through hormonal pathways, by means of the hypothalamicpituitary system (see above and standard works on endocrinology, physiology, and anatomy).
The efferent arm of the autonomic nervous system is composed of two complementary systems, the sympathetic nervous system and the parasympathetic nervous system, whose effects are generally antagonistic to each other. The efferent fibers of both systems mainly innervate the smooth muscle of the viscera, blood vessels, and glands and are thus commonly called visceral efferent (visceromotor) fibers, to distinguish them from the sensory visceral afferent fibers. The latter, unlike the visceral efferent fibers, are not divided into two systems.
General scheme of the sympathetic and parasympathetic nervous systems.
The final efferent pathway of both the sympathetic and the parasympathetic nervous systems consists of two neurons in series (Fig. 6.14). The cell body of the first (preganglionic) neuron lies within the central nervous system, while that of the second (postganglionic) neuron is found in a peripheral ganglion.
The first neurons of the sympathetic nervous system lie in the thoracic and lumbar segments of the spinal cord (intermediolateral cell column, T1L2); for this reason, the sympathetic nervous system is sometimes called the thoracolumbar system. Some of the first neurons of the parasympathetic nervous system are found in the nuclei of cranial nerves III, VII, IX, and X (see below), while the remainder are found in the lateral horns of the sacral segments of the spinal cord (pelvic parasympathetic system, S2S4). Thus, the parasympathetic nervous system is sometimes called the craniosacral system.
Baehr, Duus' Topical Diagnosis in Neurology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
6 290 · 6 Diencephalon and Autonomic Nervous System
T1
T5
T10
Oculomotor n.
N. intermedius
Glossopharyngeal n.
Vagus n.
with somatic
nerve to skin
Celiac ganglion
Greater splanchnic n.
Lesser splanchnic n.
Superior mesenteric ganglion
Ciliary ganglion
Pterygopalatine ganglion
Otic ganglion
Submandibular ganglion
Pulmonary nn. 
Cardiac nn.
Sweat |
Hair |
|
gland |
||
|
Liver
Spleen
Pancreas
Lacrimal gland Parotid gland
Salivary glands
Bronchi
Lung
Heart
Stomach
Adrenal
Kidney
Inferior mesen- |
|
|
teric ganglion |
|
Small |
|
|
intes- |
|
Colon |
tine |
|
|
|
|
(2/3) |
|
Hypogastric plexus |
|
|
|
|
Colon |
|
|
(1/3) |
|
Bladder |
and |
Pelvic splanchnic nn. |
rectum |
|
|
|
|
|
Genitalia |
|
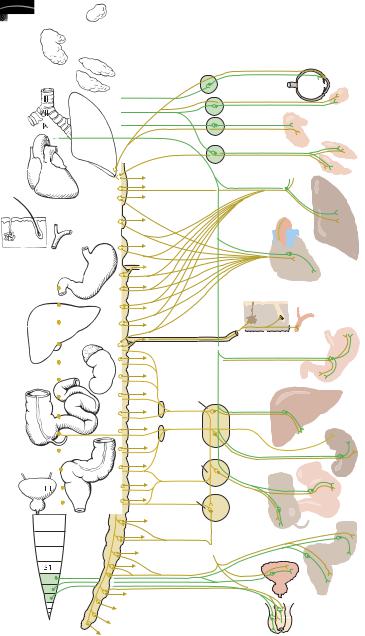
Fig. 6.14 The sympathetic and parasympathetic nervous system (schematic diagram). Yellow: sympathetic. Green: parasympathetic.
Baehr, Duus' Topical Diagnosis in Neurology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.

Peripheral Autonomic Nervous System · 291 6
The second neurons of the sympathetic nervous system are arranged in prevertebral and paravertebral chains of ganglia (the sympathetic chains), while those of the parasympathetic nervous system generally lie in the walls of the innervated organs (intramural ganglia). The first neurons of both systems use acetylcholine as their neurotransmitter. The second neurons of the parasympathetic nervous system also use acetylcholine as their neurotransmitter (a further alternative name for the parasympathetic nervous system is, therefore, the cholinergic system). The neurotransmitter of the postganglionic sympathetic neurons, however, is norepinephrine (adrenergic system). The sweat glands are an exception to this rule: the second sympathetic neuron innervating them is cholinergic, like a second neuron in the parasympathetic nervous system.
Hypothalamic control of the sympathetic and parasympathetic nervous systems. Stimulation of the rostral hypothalamus induces increased parasympathetic (trophotropic) activity, including reduction of the cardiac minute volume, hypotonia, slowing of the heartbeat, reduction of the respiratory volume, lowering of the basal metabolic rate, vasodilatation, sweating, salivation, contraction of the bladder, reduced secretion of epinephrine, increased peristalsis, and pupillary constriction. Stimulation of the caudal hypothalamus, on the other hand, induces increased sympathetic (ergotropic) activity, including a rise in blood pressure, acceleration of the heartbeat, increased blood supply to the skeletal muscle and lungs, vasoconstriction in blood depots such as the capillary bed of the digestive tract, decreased blood supply to the abdominal viscera, increased respiratory volume, a rise in the blood glucose level, inhibition of peristalsis, urinary retention, increased secretion of epinephrine, widening of the palpebral fissure, and pupillary dilatation. A mass reaction thus occurs in the entire body, directed toward physical exertion and therefore enabling the whole organism to deal optimally with situations of attack and stress. While the sympathetic, ergotropic reaction is directed toward physical exertion, the parasympathetic, trophotropic reaction is directed toward rest and recovery. Despite these general principles, however, the distinction between parasympathetic and sympathetic activity is not always clear-cut.
Neural connections of the hypothalamus to the peripheral autonomic nervous system. The hypothalamus exerts its regulating and controlling functions over the sympathetic and parasympathetic nervous systems by means of descending pathways including the medial forebrain bundle (Fig. 6.9), the mamillotegmental tract, and the dorsal longitudinal fasciculus (of Schütz) (Fig. 6.10).
Baehr, Duus' Topical Diagnosis in Neurology © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.
