
The Art of Genes How Organisms Make Themselves
.pdf
order of expression of the genes in vertebrates was the same as in flies, with apple-green being nearest the head end, followed by bottle-green, then cyprus-green, and eventually ending up with herb-green towards the rear. Together, the various greens gave a series of distinct regions going from head to tail, much as had been observed for fruit flies.
Fig. 7.6 Diagrams representing mouse embryos stained to reveal the expression pattern of a gene needed for bottle-green (left) or cyprus -green (right). Note that the region of cyprus-green starts further back than bottle-green (see arrows).
This was a startling result because it showed that there was a common aspect to the organisation of insects and vertebrates: they share an underlying map of colours that follow each other in the same relative order from head to tail. It is reminiscent of Geoffroy's principle of connections, but here it applies to hidden colours rather than bones. Whereas Geoffroy's rule was based on the order of parts of the skeleton, in this case it is the order of hidden colours that is held in common. Vertebrates and insects are united by a set of green hidden colours that are connected or ordered in a similar way from head to tail.
There is still the question, however, of what this map of green hidden colours signifies for vertebrates. In flies, they establish territories in the animal from head to tail, conferring distinctions between the various segments. Take away one or more of the colours, through mutations in the genes, and the identities of some segments are no longer distinguished. Perhaps the hidden colours also provide distinctions between the different parts of vertebrates. Although vertebrates are not segmented in the same way as insects, they are made up of repeated elements of various types from head to tail. This is most obvious in the backbone, which is made of a series of repeating units or vertebrae (Fig. 7.7). Going from head to tail in humans there are seven vertebrae in the neck region (cervical vertebrae), followed by twelve vertebrae in the chest region, bearing ribs of various lengths (thoracic vertebrae), followed by another five vertebrae without ribs in the abdominal region (lumbar vertebrae). Beyond this, there are several other vertebrae at the base of the back. In mammals with tails, the vertebrae can continue much further than this. The various types of vertebrae from head to tail are the most obvious place to look for a comparable role of the green hidden colours in vertebrates: perhaps the green colours provide distinctions between vertebrae from head to tail, similar to the way they distinguish between insect segments.
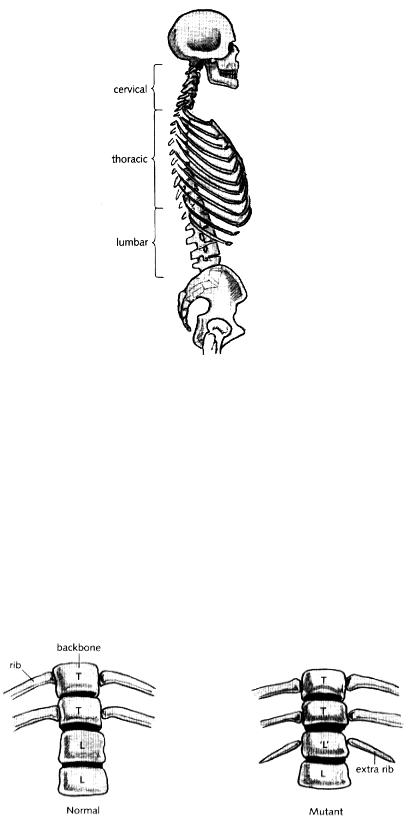
Fig. 7.7 Human backbone in side view showing the various vertebrae with and without ribs.
If the hidden green colours are important in providing distinctions between vertebrae, mutants that lack one of the colours might be expected to show alterations in the identity of particular vertebrae. This could be tested in mice because a method had been developed that allowed specific mouse genes to be inactivated by mutation. This method was used to inactivate particular homeobox genes, producing mutant mice that lacked the corresponding hidden colours. Some of these mutants did indeed show striking changes in the identities of specific vertebrae. An example is shown in Fig. 7.8, taken from the work of HervéLe Mouellic, Yvan Lallemand and Phillipe Brûlet, working in Paris in 1992. As in humans, mice have a series of thoracic vertebrae, each bearing a pair of ribs, followed by a series of lumbar vertebrae that do not have ribs attached to them. The region of transition from thoracic to lumbar vertebrae is illustrated for a normal mouse in the left part of Fig. 7.8.
Fig. 7.8 Diagram of part of the skeleton of a normal mouse (left) showing thoracic (T) and lumbar
(L) vertebrae, compared to a mutant (right) which carries a mutation in a homeobox gene contributing to the grass-green colour. Note the extra pair of ribs in the mutant that partially transforms a lumbar ('L') vertebra into a thoracic vertebra.
The diagram on the right of Fig. 7.8 illustrates a mouse which has a mutation in one of its homeobox genes, a gene contributing to the grass-green hidden colour. In this mutant animal, the first lumbar vertebra— normally lacking ribs— has a pair of small ribs attached to it. The lumbar vertebra is displaying a feature, a pair of ribs, normally associated with the thoracic vertebrae that lie ahead of it in the backbone. Indeed, given that thoracic vertebrae are defined as those that bear ribs, we might say that the first lumbar vertebra has been replaced by a thoracic vertebra: its identity has changed from lumbar to thoracic. In other words, as with flies, the hidden colours are needed to provide distinctions along the head-tail axis, in this case between vertebrae. Mutations giving a loss of colour lead to a lack of distinction, such as a lumbar vertebra assuming a similar identity to a thoracic vertebra. You will notice that the transformation is not complete: the extra set of ribs is much smaller than the ribs that come off a normal thoracic vertebra. This is most probably because there are four copies of the homeobox gene cluster in mice, which can act to some extent as backups for each other. Mutation in a gene from one duster will, therefore, not completely eliminate a hidden colour, such as grass-green, because there are genes in the other dusters that can act to some extent as substitutes.
The analysis of mutations in other homeobox genes gave similar results. Mutant mice that lacked a homeobox gene contributing cyprus-green had alterations in their cervical vertebrae, resulting in the second neck vertebra assuming an identity more like that of the first vertebra.
I have emphasised vertebrae because they are the easiest feature to look at with respect to the head-to-tail axis of vertebrates, but the hidden green colours also affect other distinctions along this axis. In other words, the set of green hidden colours provide distinctions between territories from head to tail that will give rise to various structures during development, of which the vertebrae are the simplest example.
To summarise, as with flies, the green hidden colours of vertebrates are needed to provide distinctions between various regions from head to tail of the animal. Loss or reduction in one of these hidden colours results in a lack of distinction between regions along the head-tail axis, most easily seen as some vertebrae that are normally quite distinct starting to assume similar identities. Thus, the anatomical layout from head to tail in vertebrates depends on an underlying map of green hidden colours similar to that found in insects.
From colour to anatomy
If insects and vertebrates contain a similar set of green hidden colours, how come they end up looking so different from head to tail? Recall that the hidden colours do not correspond to a set of instructions that specify how a structure should be made, like the construction of a particular type of segment: they simply provide a frame of reference that is then interpreted by genes. So although two organisms may have a similar map of green hidden colours, the way this is interpreted and eventually becomes manifest in the anatomy of the animals can be very different.
We are still very far from understanding the precise relationship between hidden colours and the final anatomy of any animal, be it a vertebrate or an insect. Nevertheless, I shall try and outline
some of the contributing factors to give a better idea of why these animals can end up looking so distinct, even though they share a similar underlying map.
In Chapter 5, we saw that hidden colours correspond to master proteins that can bind to particular sites in the regulatory regions of interpreting genes. The emerald-green master protein, for example, may recognise one short sequence of DNA, an E-site, whereas the grass-green protein recognises a slightly different sequence, a G-site. By binding to these sites, the master proteins can influence whether an interpreting gene is on or off. In the simplest scenario, an interpreting gene with an E-site in its regulatory region will be switched on wherever the emerald-green protein is to be found in the organism. Similarly, a gene with a G-site will be expressed wherever the grass-green protein is located. Now although vertebrates and insects have a common map of green master proteins along the head-tail axis, many of the interpreting genes that respond to each master protein might be different in each case. We can imagine, for example, that many of the interpreting genes with an E-site in insects may be quite different from those with an E-site in vertebrates. During the long evolutionary time that has separated vertebrates from arthropods, the sites in the regulatory regions of interpreting genes could have changed. This would mean that the interpreting genes expressed in the emerald-green protein territory in vertebrates would differ from those expressed in the emerald-green territory of insects. The map of colours may be the same but their interpretation would differ.
The situation is, of course, much more complicated than my simplified scenario implies. For one thing, interpreting genes have several binding sites in their regulatory regions, allowing them to respond to a combination of hidden colours in the organism. There are many other types of master protein, in addition to the green family, each contributing a hidden colour to give a complex overlapping patchwork. The response of a gene to one or more green colours has to be set in the context of these other hidden colour patterns, some of which might be similar between vertebrates and insects, whereas many others might differ. Furthermore, the proteins encoded by the interpreting genes may themselves have evolved so that their effects on the organism can be different in insects and vertebrates. The way the interpretation of the hidden colours eventually leads to the visible structures of the animal is a very complex affair that can differ between insects and vertebrates in numerous ways.
All of this means that the process by which a set of hidden colours is interpreted and eventually becomes manifest in the final anatomy of the organism is likely to change in many ways during evolution. In insects, the green hidden colours eventually become manifest in the various types of segments, whereas in vertebrates their effects become apparent in other structures, such as the types of vertebrae. So even though the head-to-tail layout of insects and vertebrates depends on a similar map of hidden colours, you might never guess this on the basis of their anatomy alone.
A hidden unity
We can now take another look at the debate between Geoffroy and Cuvier. Cuvier's emphasis on function led him to see insects and vertebrates as being constructed on entirely different principles. There was no meaningful way of comparing these two types of animal. Geoffroy on the other
hand was struck by the similar arrangements of parts in groups of animals, such as the mammals, irrespective of what particular function the parts served. Having revealed what he believed to be an underlying law of similarity, the way the bones were connected together, he tried to extend this law to other animals, going as far as insects. To do this he had to establish some sort of correspondence between the parts of an insect and a vertebrate. His solution was to match the outer skeleton of insects with the inner skeleton of vertebrates. Once this was done, detailed correspondences could be drawn, such as between the vertebrate backbone bearing ribs and the outer body of the insect bearing legs. Both systems could be seen to fall under the same umbrella of connections.
With the benefit of hindsight, we can see that there were some merits to Geoffroy's view. There is an underlying set of connections that is similar between insects and vertebrates: the map of hidden colours. Both types of animal have a family of green hidden colours that are arranged or connected in the same order from head to tail, apple-green at the head end, through to herb-green at the tail end. In both cases, the colours provide regional territories, leading to structures with distinct identities developing along the head-tail axis. However, the way that this common map is interpreted and eventually becomes manifest in the visible features of the organism is quite different in each case. In insects, it becomes manifest as a distinction between the various types of segments of the animal whereas in vertebrates it affects different structures, most notably the types of bones arranged along the backbone. Anatomy therefore reveals this underlying map of hidden colours only indirectly: through the way the map is interpreted and eventually becomes manifest in the animal.
It follows that even if animals have a similar set of underlying colours, this unity might only be dimly perceived at the level of their anatomy. That is why many of Geoffroy's detailed correspondences, such as between vertebrate ribs and insect legs, proved to be unconvincing: he was trying to establish a unity of plan based purely on anatomical features. In this sense Cuvier was right to disparage some of Geoffroy's comparisons. Yet although we might fault Geoffroy on some of his particular claims, his overall insight that there was a common underlying map that unified animals as different as insects and vertebrates did prove to be correct.
From an evolutionary point of view, these limitations of anatomical studies can be seen to depend on the degree of relatedness between the animals being compared. There is no difficulty, even from the most superficial inspection, in establishing the correspondence between a human hand and the hand of an ape. It is more difficult to see the relationship between a human arm and a horse's leg or a whale's flipper. In these cases, if we only look at them from the outside, we might conclude that they are entirely different types Of appendage. The correspondence is greatly clarified, however, by looking at the arrangement of bones in the skeleton. What looks superficially different is seen to reflect a common arrangement of bones. But when we get to more distantly related animals, such as insects and vertebrates, even anatomical comparisons become of limited use. Based on these, we might have reasonably concluded that the basic layout of these animals had nothing in common (unless, like Geoffroy, we believed in a fundamental unity of plan). The anatomical layouts could have evolved completely independently of each other. To get a deeper insight, we need to look at the map of hidden colours that underlies the anatomy of the
organism. Then we see that there is a unity even though it is interpreted and becomes manifest in very different ways in the anatomy of the animal. The map provides a hidden skeleton, a set of underlying connections that does allow meaningful comparisons between very diverse organisms to be made.
I do not mean to imply that the green hidden colours provide an absolute or immutable map. There have been alterations in the homeobox genes needed for the green colours during evolution, such as extra duplications of individual homeobox genes. Even entire dusters of homeobox genes have been duplicated to give the four dusters present in mice and humans of today. These alterations may have allowed the map of green colours to have been modified to some extent. Nevertheless, the basic order of green hidden colours from head to tail, the connections in the map, does not seem to have changed in a fundamental way during the six hundred million years that separate insects and vertebrates from their common ancestor.
What role might the homeobox genes have had in this common ancestor of insects and vertebrates? It seems most likely that, as with organisms alive today, its homeobox genes would also have coded for a set of hidden colours that gave distinctions from head to tail. In this case, however, the various territories of colour may not have been interpreted to give distinctive segments, like those of flies, nor different type of vertebrae, as in mice. Rather, they could have been manifested in a different way again, as I shall now explain.
A good illustration comes from the study of homeobox genes in the nematode Caenorhabditis elegans, a tiny worm-shaped animal (about one millimetre long) that belongs to a quite distinct group (phylum) from insects or vertebrates. This worm has neither segments nor vertebrae, yet it still has a duster of homeobox genes (the worm has only a set of four genes in its cluster as compared to the eight or more observed in insects and vertebrates). As with the other animals I have mentioned, each worm homeobox gene is expressed in a distinct region of the animal, producing territories of green hidden colour from head to tail. The hidden colours are then interpreted by genes so that different parts of the worm assume distinct identities along the head-tail axis.
In this case, though, the identities do not refer to segments or vertebrae, but to groups of cells that are repeated along the worm's length. These cell groups are not surrounded by a hard outer casing, like insect segments, nor do they produce bony regions like vertebrae; they simply form characteristic regions of the worm. In other words, the hidden colours still provide a map or frame of reference from head to tail, but this is interpreted and eventually becomes manifest differently for the worm than for vertebrates or insects.
We can conclude that the common ancestor of insects and vertebrates (and nematode worms) most likely had a cluster of homeobox genes that provided distinctive identities along its head-tail axis. However, the way this hidden map was interpreted and became manifest in this early animal was probably quite different from what we see in humans or flies today. There is a remarkable unity in the map of some hidden colours between animals, which has been preserved for hundreds of millions of years; yet the way this becomes manifest in their anatomy can be very different. We
shall return to the question of why such a deep unity can be discerned with hidden colours in some of the chapters towards the end of this book. In the next few chapters I want to deal with a fundamental problem that I have so far skipped over: how the pattern of hidden colours is itself established during development.
Chapter 8 The expanding canvas
So far in this book, I have described how the development of organisms depends on a patchwork of hidden colours This patchwork provides a frame of reference that can be interpreted by numerous genes (interpreting genes), allowing them to be expressed at particular times and places in the organism This pattern of gene activity, in turn, underlies the complex anatomy of plants and animals But I have yet to explain how the patchwork of hidden colours itself originates I have taken it for granted that the patchwork is there without explaining how it came about
In the next few chapters, I shall try to deal with this problem I will show that the pattern of hidden colours arises through a chain of events, involving one set of hidden colours building on another set of hidden colours, which in turn depend on another set In the present chapter I want to give a broad sense of what is being achieved as hidden colours build on each other in this way To do this, I will draw an analogy between establishing a patchwork of hidden colours and painting a picture In both cases, a complex arrangement of distinctive regions is produced: in the case of a painting, this is done within the confines of a two-dimensional canvas; whereas in biological development, the territories of hidden colour are distributed within the three-dimensional framework of the organism In following this analogy, I shall have to introduce some bizarre notions, like canvases that grow and expand or artists that multiply and proliferate Although these images may seem fanciful at first sight, their purpose is to highlight some of the salient features of development
Refining a pattern
There are many different ways of painting a picture. Perhaps one of the most systematic approaches is painting by numbers: a picture is carefully drawn out and each region labelled with a number to indicate where each colour should go. All the spatial information is already there in the drawing; the act of painting just transforms numbered regions into coloured ones.
The trouble with painting by numbers as an analogy for the origin of a hidden patchwork is that it does not add any new spatial information. If we were to say that each territory of hidden colour arose by filling in a series of numbered regions, it would simply beg the question of where the numbered regions came from. Indeed, in the sense I am using hidden colours— as a way of distinguishing one territory from another— they are essentially no different from numbered regions (although they are more convenient than numbers for conveying territories). We would be trying to account for one set of territories simply by reference to an identical set of underlying territories that is coded in a different way, hidden numbered regions rather than hidden colours. It takes us no further because the origin of the numbered regions is just as difficult to explain as the origin of the colour patterns.
There is another style of painting which provides a much more useful analogy than painting by numbers. Look at Fig. 8.1, which shows three stages during a self-portrait, taken from a teach-yourself book called Creating a Self-Portrait. In the first stage, the artist has broadly defined

a few regions of colour, showing approximately where the face, hair, and shirt will go. At this stage, the outlines of each region are somewhat blurred and crudely defined. In the second stage, colours have been laid over the first ones to provide more detail: the position of the eyes, nose, mouth, shirt collar, etc. The first stage has acted as a broad framework that has then been refined during the second. This process of refinement has continued even further in the third stage, which now shows details such as the eyes' pupils, nostrils, chequered markings on the shirt, etc. We can see that each stage is a response to what went before, with colours being added to build up and refine the picture, the brush strokes becoming finer and more detailed at each step. This is how one of the other artists mentioned in the same self-portrait book, Roy Freer, describes his method:
My approach to watercolour painting is very similar to that with oil or pastel. I start with a broad description of the essential masses and then progress through several stages of refinement until I have achieved the final result.
Fig. 8.1 Three stages in a self-portrait by Francis Bowyer (from Coates 1989).
Why doesn't the artist go directly to the detailed last stage, circumventing the other steps? What purpose do the previous stages serve? One problem with going too quickly into painting details is that they can be easily misplaced. If you start by painting the eyes in great detail, you might find later on that they are not in quite the right place in relation to the other features. You would have to go back and start all over again. By first giving the overall layout and gradually narrowing down to the details, the correct relations between features can be established gradually at each scale, from the overall location of the face, to the position of eyes, to the details within each eye. To help them achieve this, some artists screw up their eyes when looking at the subject during the early stages of a painting, blurring their vision on purpose so as to more easily see the broad arrangements and avoid being distracted by details.
Basing one set of colours on a previous set in this way can help to refine the details of a picture. We shall see that this provides a good analogy for how hidden patchworks arise during biological development. The pattern of hidden colours is built up and refined gradually as an organism develops rather than being simply applied in one step to a pre-existing framework, as occurs in painting by numbers. Hidden colours are established through an interactive process in which one set of colours depends and elaborates on what went before.
The growing canvas
There is an important aspect of internal painting that is not captured by the visible painting process as I have described it so far: organisms can grow at the same time that their patterns are being refined. It is as if the canvas is not of a fixed size but it is continually enlarging. The extent of growth that can occur during development is quite remarkable. Here is how the eighteenth-century biologist Albrecht yon Hailer described the situation:
The growth of the embryo in the uterus of the mother is almost unbelievably rapid. We do not know what its size is at the moment of its formation, but it is certainly so small that it cannot be seen even with the aid of the best microscopes, and it reaches in nine months the weight of ten or twelve pounds. In order to dear up this speculation, let us examine the growth of the chick in the egg. We cannot in this case either measure its size at the moment when the egg is put to incubate but it cannot be more than 4/100 in. long, for flit were, it would be visible, and yet 25 days later it is 4 ins. long. Its relation is therefore as 64 to 64 millions [in weight] or 1 to 1 million.
Since Haller's time, the details of human embryonic growth have been established. At the time of fertilisation, the size of a human egg is about 0.1 mm in diameter and it weighs about one-millionth of a gram. After about seven weeks, it has become about 17 mm long with a weight of one gram: an increase in weight of one million fold. By the time an adult is formed, the size will have increased by a further factor of about one hundred in linear dimensions and one hundred thousand in weight. Overall, the growth from fertilised egg to adult will involve an increase by factors of about ten thousand in linear dimensions and one hundred billion in weight. Similar calculations can be made for plants. A typical plant egg cell is of the order of one-hundredth of a millimetre in diameter, yet it may grow to form a tree more than ten metres tall with roots perhaps extending for a comparable distance underground: an increase of about one million fold in linear dimensions. We therefore need to think of hidden colours being built up within a growing framework rather than one of fixed size.
In terms of the painting analogy, we can imagine the canvas expanding whilst the painting proceeds. I have illustrated this in Fig. 8.2 by showing the same three stages of the portrait in Fig. 8.1, but now with their overall size increasing as they go through the various stages. Between each stage, I have enlarged the portrait by a factor of 4 in linear dimensions, corresponding to a factor of 16 in area, giving a total enlargement over the two stages of 16 times in linear dimensions, or 256 times in area. Of course in this case, growth only occurs in two dimensions, whereas an organism grows in three dimensions.
