
The Art of Genes How Organisms Make Themselves
.pdfto our dog. A geometrical transformation, such as reflecting an object in a mirror, seems like a mystical notion that is hard to grasp in comparison to a change in smell brought about by a chemical reaction. Compared to us, the dog is weak in geometry but strong in chemistry.
Now when it comes to understanding development, our dog may have a few advantages. It seems to me that many of the difficulties we have in thinking about development stem from our over-reliance on external geometry. To us, the most striking feature of an egg developing into an adult is the increase in geometrical complexity: an egg is more homogeneous in terms of spatial organisation than the adult that eventually develops from it. Yet from a purely geometrical perspective this seems unintelligible, because the basic transformations in geometry applied to an object do not increase complexity but preserve complexity. Take a simple geometrical transformation, such as moving an object in a straight line from one place to another, a process mathematically called translation. Although this changes the object's position, it does not change its complexity in any way. Or consider other geometrical changes, like rotating an object, or reflecting it in a mirror, or magnifying or reducing its size. Although all of these operations change the object's appearance in some way, they do not change its quality or complexity. We simply end up with the same object presented from a different viewpoint.
In my view it is this geometrical perspective that draws us towards the idea that there is a preformed version of the adult in the egg (Chapter 1). Preformaion is an attractive way of explaining development because it accounts for the whole process as a series of purely geometrical transformations: enlargements of a miniature that is just as complex as the fully grown adult. It is easy to comprehend because it appeals to our visually dominated mind.
However, in trying to understand development we need to get away from this purely geometrical viewpoint, and think of the transformations more in terms of their underlying chemistry. We need to think more like our dog: through our nose rather than our eyes.
Now I seem to have boxed myself in here. I am suggesting that we should try and think more like a dog; yet we are fundamentally visual animals. We cannot simply change the way we perceive the world by starting to think in terms of odours rather than images. We comprehend things much better through getting a mental picture of what is going on than by forming some sort of internal smellogram. That is why we commonly acknowledge that we understand something by saying 'I see what you mean' rather than 'I smell what you mean'.
I shall try to deal with this problem in very much the same way that a chemist might, by still using the concepts of geometry but applying them at the molecular level rather than at the level of what is externally visible. Much of our understanding of chemistry is based on the twin notions of molecular geometry and energy. We think of a chemical reaction in terms of a redistribution of energy as the atoms in molecules are rearranged in space. Rotten eggs give off a horrible smell because of a chemical reaction in which hydrogen and sulphur atoms combine in a particular spatial arrangement to form a pungent gas called hydrogen sulphide. What is perceived at one level as a change in the properties of a chemical, such as its smell, can be thought of at the submicroscopic level as a rearrangement of atoms, a change in molecular shape (recall that I am using shape to refer to the distribution of electric charges as well as the overall three-dimensional arrangement of atoms). A change in smell can give us an overall intuition that a reaction is going on, but to get a more exact idea of what is happening we have to turn to molecular geometry.
In this chapter, I shall use the notion of smell to help explain a key aspect of development: how cells communicate. Just as I used colours to give an intuition of how territories in an

organism are defined, so smells will now help to take the process further by conveying how cells interact with each other. As with the colour metaphor, I shall use smell to give an overall idea of the process, whilst at the same time providing a more precise explanation of what is happening in terms of molecular shapes and interactions. We will get a more dog-like overview (or oversmell) but it will be based on a foundation of molecular geometry.
Although I shall not talk about the detailed energy changes involved in these molecular events, you should bear in mind that all of the processes require energy, stored in the form of chemical bonds, to drive them along. As described in Chapter 2, this energy ultimately comes from the sun. It is through channelling this energy that more geometrical complexity can eventually emerge as development proceeds.
Receptor proteins
A good point to begin our exploration of smells will be to take a closer look at our nose. The nose is not confined to the visible protuberance on our face, but penetrates inwards for several centimetres. A group of sensory cells lying deep in this cavity detect smells and send the information they receive to the brain. The sensory cells can recognise molecules in the air because of particular types of protein in their membranes, called receptor proteins. It is the shapes of these proteins that allow us to distinguish between different chemicals entering the nose. Each receptor protein sits in the membrane of a sensory cell and points into the nasal cavity, where it may encounter molecules that are inhaled along with air (Fig, 11.1). There are hundreds of different types of these receptor proteins in the nose, each with a shape that matches a particular range of molecules. If a molecule matches or binds to a receptor protein, it will influence the shape of the protein. I have indicated this in the figure by showing a bump forming at one end of the protein. Recall that the shape of a protein determines what it does — the sorts of reactions it encourages to happen. So when the receptor protein changes its shape, it facilitates a new chemical reaction in the cell. This in turn sets off a chain of other reactions, eventually leading to an electrical nerve impulse being sent from the sensory cell to the brain.
Fig. 11.1 Receptor proteins in the sensory cells of the nose are triggered when molecules of scent bind to them, setting off a chain of reactions.
How does the brain know which particular receptor proteins have been triggered when we smell something? Although many different types of receptor protein are produced by the sensory
cells of the nose, each individual sensory cell is thought to make only one or very few types of receptor protein. So when we sniff cinnamon, the inhaled molecules will only trigger a particular set of cells, those that have receptor proteins matching cinnamon. These then send a set of nerve impulses to the brain. It is this combination of impulses, coming from a particular set of sensory cells in the nose, that gives us the sensation of having smelled cinnamon. Sniffing almonds will stimulate a different combination of sensory cells, which we register as the smell of almonds. Even humans, with their relatively undeveloped sense of smell, are thought to be able to distinguish between about ten thousand different odours in this way. We can distinguish more smells (thousands) that the number of different types of receptor protein (hundreds). This is possible because the inhaled molecules do not trigger just one type of receptor protein but several different types. It is the combination of receptor proteins triggered by a molecule that provides the molecule with its distinctive chemical signature.
Not everyone is able to smell things to the same extent. After eating asparagus, some people will describe a very strong smell in their urine, whereas others will not detect anything remarkable. In a series of tests, in which people sniffed urine collected from a man after he had eaten 450 grams of canned asparagus, it was shown that some people were very sensitive to the smell whereas others were not. It seems that everyone produces pungent compounds in their urine after eating asparagus but some people can't smell them very well. Similarly, some people are insensitive to the smell of freesia flowers. The insensitivity to particular smells is called specific anosmia, the olfactory equivalent to being colour-blind.
The cause of specific anosmias is not yet known but in several cases they have been shown to be inherited. That is to say, they are passed on by genes. A good guess is that some of them result from mutations in genes for receptor proteins. Like all other proteins, each receptor protein in the nose is coded for by a gene. If someone has a mutation in one of these genes, a change in its DNA sequence, their corresponding receptor protein may be defective. This would in turn lead to a reduced ability to detect particular molecules, such as those in the scent of freesias. Thus our sensitivity to smells depends on our repertoire of genes that code for receptor proteins.
Our sense of smell gives us direct information about molecules in the air because it employs receptor proteins that can discriminate between their shapes. When a molecule binds to a receptor protein in the nose, it sets off a chain of chemical events in the cell that leads to a particular response, the sending of a nerve impulse. Our ability to smell can be understood in terms of geometry: the geometry of how atoms and charges are arranged to give molecules their shape. That is how smell can monitor the molecules that are wafted into the air as a cake is baked, as milk turns sour or as an egg turns rotten.
Taste is thought to operate in a similar way to smell, though in this case there are far fewer types of receptor involved. We can only discriminate between four types of taste (sweet, salty, sour and bitter) through sensory cells in our tongue. Much of what we commonly call taste is actually due to us smelling the food while we eat it (hence our inability to taste much when we have a blocked nose).
Smell thy neighbour
As well as allowing organisms to smell or taste their external environment, receptor proteins also play a fundamental part in communication between cells within the body. In the case of our
nose, the molecules that are detected by receptor proteins come from the surrounding air. Most cells in our body, though, are not surrounded by air but by other cells. Even the sensory cells of the nose have air only on one side of them and are surrounded on other sides by cells. We shall see that receptor proteins provide a very important mechanism that allows cells to respond to their neighbours.
In Chapter 9, we saw that the refinement of patterns in the early fly embryo depended on communication between neighbouring nuclei. Without some sort of communication, each nucleus would go off and do its own thing irrespective of its neighbour, giving a hotchpotch rather than a coordinated elaboration of a pattern. In this case there was no problem with communication because there were no cell membranes that separated the nuclei. The nuclei were immersed in a common cytoplasm so hidden colours (master proteins) could diffuse from one region to another.
This, however, is a rather exceptional situation. For the most part, development occurs while nuclei are located in separate cells. Master proteins cannot diffuse freely from one cell to another because of the cell membranes that are interposed between them. This has two important consequences. On the one hand, it means that diffusion is not continually mixing up hidden colours from neighbouring regions, so that the patterns can be more stable and distinct: the hidden colour in one cell will not be continually leaking into its adjacent cells, so it is easier to keep territories of hidden colour separate. On the other hand, there is no longer a straightforward means of communication between neighbours: nuclei are no longer in free contact with each other so they cannot coordinate their activities through diffusion. But without some sort of contact, the system would tend towards cellular anarchy, with each cell doing its own thing.
This is where receptor proteins come in: they allow cells in the developing organism to communicate with each other even though they are separated by membranes. Patterns can be refined in a coordinated way because cells can send molecular signals to each other via receptor proteins. We shall see that unlike our true sense of smell, triggering receptor proteins during development does not lead to nerve impulses being sent to the brain, but to a different sort of response— typically a change in hidden colour. It is as if cells are continually sniffing each other, modifying their hidden colours according to the scents they detect.
Induction in newts
One of the first pieces of evidence that chemical communication between cells might be important for development goes back to the 1920s, to Hans Spemann and his school in Germany, working on newts. Spemann was taking small pieces from one newt embryo and implanting them in another to see how the implanted tissue might develop. In most cases, he found that if the transplantation was carried out at an early stage of development, there was little disturbance in development: the implant joined in the development of its host. However, he noticed that when a particular region of the early embryo was transplanted, there was a dramatic effect. The implant did not simply join in the development of its new surroundings; on the contrary, a second embryo formed at the site of implantation. It seemed that the transplantation of this particular region could result in the formation of an almost entire second embryo.
At first Spemann thought this second embryo was derived simply by the growth and development of the implant. However, a few years later he changed his mind, when his collaborator Hilde Mangold repeated the experiments using two newt species with embryos that

could be easily distinguished by virtue of their different pigmentation (Fig. 11.2). By taking a piece of embryo from a lightly pigmented species and transplanting it into a darkly pigmented host, the implant could be permanently recognised by its lighter colour. Spemann and Mangold then saw that when a secondary embryo formed, it was not just made from the light-coloured implanted tissue but also contained large amounts of darkly pigmented host tissue. It was as if the implant had somehow organised or induced its nearby cells from the host to participate in producing a new embryo.
Fig. 11.2Experiment by Spemann and Mangold in which part of an early newt embryo from a lightly pigmented donor was implanted in a darkly pigmented host. This induced the formation of a second embryo containing large amounts of host tissue
A possible explanation was that the implant was sending some sort of chemical signal that induced the nearby cells to behave in this way. If the chemic al could be identified, the nature of how cells coordinate their activities during development might be revealed. A crusade followed to try and identify the chemical, but unfortunately this met with more frustration than success. Joseph Needham summed up the situation in 1939:
In conclusion, it may be said that although the progress made in the last ten years in these fields has been very great, we can nevertheless see now that owing to the special difficulties of the subject ... it may be more like fifty years before we can expect to have certain knowledge concerning the chemical nature of the naturally-occurring substances involved in embryonic induction. Like so many other biological problems, this has turned out to be more complex than the first explorers thought.
Needham's estimate of fifty years was not too far out. It was only in the late 1980s and early 1990s that the nature of these sorts of chemical signal involved in development started to be unequivocally identified. An important turning point came from molecular studies on specific genes, as I shall now illustrate with an example taken from fruit flies.

A molecular marriage
During the 1960s, Seymour Benzer's laboratory at Caltech was screening fruit flies for mutants that did not res pond much to light. If you shine light on normal flies, they tend to move towards the light source. Benzer was looking for exceptional individuals that did not behave normally and stayed put instead of wandering over to the light. One of the mutants he obtained was later shown to have a specific defect in its eyes, effectively making it partially blind to the light.
To understand the defect in Benzer's partially blind flies, you need to know some background about insect eyes. The eye of a fruit fly is made up of about 750 identical facets or ommatidia (Fig. 11.3). Each ommatidium contains 20 cells of various types, each cell type being specialised in a different way: one cell type secretes the main lens, another produces pigment, and yet other cell types are responsible for detecting the light. These 20 cells are arranged in a very precise and stereotyped way, with the same pattern of cells being repeated in every ommatidium. I am going to concentrate on the interaction between just two of these cells, called R7 and R8.
Fig. 11.3 The eye of a fruit fly is made up of many ommatidia. Some of the cells inside one ommatidium are show n so as to reveal the relative positions of R7, R8 and cone cells.
One of Benzer's mutants was partially blind because it lacked a particular type of cell from every ommatidium: the R7 cell. Without the R7 cell in its ommatidia, the fly could not see properly and therefore did not respond to light in the same way as normal flies. Because of this deficiency in R7, the mutant was named sevenless. The striking thing about the sevenless mutant is that apart from lacking R7, the ommatidia otherwise seem to be normal. To get a graphic illustration of what this means, look at the painting by Magritte shown in Fig. 11.4. It shows one room with a man reading a paper, and three other rooms that are identical except that they are unoccupied. We can think of the eye of a normal fly as equivalent to 750 identical rooms (ommatidia), each with a person in it (the R7 cell). The eye of the sevenless mutant would then be like an identical set of rooms with the man missing in each case. In the sevenless mutant, one element is lacking, the R7 cell, but otherwise the pattern of cells in each ommatidium is unchanged; just as in the empty rooms of the painting, only the man and his paper are missing while the rest remains unchanged. It is a very specific change in the pattern, affecting one element independently of the others. Strictly speaking, the cell that would have become R7 is not really missing in sevenless mutants, but has become like one of the other types of cell normally found in the ommatidium, called a cone cell. Everywhere an R7 cell should have been made, a cone cell develops by default. In the same way, in Magritte's painting, the area of the canvas occupied by the man has not been cut out in the empty rooms but has been replaced by elements from the background, suc h as wall and floor.

Fig. 11.4 Man with a Newspaper (1928), Rent Magritte. Tate Gallery, London.
The sevenless mutant flies carry a defect in one particular gene. By convention, this gene is given the same name as the mutant— sevenless. But to avoid confusion between the mutant and the gene, from now on I shall refer to the gene by the abbreviation sev, only using the full name sevenless when referring to the mutant fly. Thus, a normal fly has a functional sev gene allowing the development of an R7 cell in each ommatidium. In sevenless mutant flies, the sev gene is defective and as a consequence of this, the R7 cell does not form (a cone cell forms instead). That is to say, the normal significance of the sev gene is to promote the formation of an R7 cell; so that when sev is defective, R7 is missing. You should be clear that although the sev gene only influences the R7 cell in each ommatidium, the gene is also present in the DNA of every other cell in the body. It is just that the only significant effect of the sev gene is on the development of one particular type of cell, R7.
I now want to look at how the sev gene exerts its effect on the development of the eye. Although the sevenless mutant highlights the normal significance of the sev gene— its requirement for the development of R7— it does not tell us how the sev gene actually works. How does a
functional sev gene normally lead to the development of an R7 cell? Like other genes, the sev gene codes for its own particular type of protein, and to appreciate how it works, we need to know what this protein does. An important breakthrough in addressing this issue came in 1987, when Ernst Hafen, Gerry Rubin and colleagues, working at Berkeley, were able to isolate the sev gene. They could then show that the protein encoded by sev had a structure that was typical of receptor proteins. Remember that receptor proteins are able to recognise and bind to specific molecules coming from outside the cell. Once a receptor protein has been triggered by binding to a molecule, it changes in shape, setting off a chain of chemical events within the cell. The sev gene codes for a particular protein of this type, that I shall from now on refer to as the sev-receptor protein. Finding that the sev gene coded for a receptor protein implied that some sort of molecule existed, equivalent to a scent, that was triggering the receptor. However, unlike what happens when we smell something, the response to the scent was not the sending of a nerve impulse to the brain, but a developmental event: the formation of an R7 cell in each ommatidium. In flies without the sev-receptor protein (sevenless mutants) there would be no such response, so the R7 cell would fail to develop.
The question now became: what was the nature of the scent, the molecule that the sev-receptor protein was detecting? An important advance in answering this came in the late 1980s, with some studies by Lawrence Zipursky and colleagues at the University of California, Los Angeles. These researchers found another type of mutant fly that lacked R7 cells, which they called bride-of-sevenless (the reason for this curious choice of name will soon become apparent). In this case, the defect was not in the sev gene but in a different gene, which I shall refer to as boss (the abbreviation of bride-of-sevenless). Like sev, the boss gene is needed for the R7 cell to develop: in bride-of-sevenless mutants, the boss gene is defective, so R7 is missing. There are therefore two different types of mutant which lack R7— sevenless and bride-of-sevenless— each due to a defect in a particular gene, sev or boss. But whereas the sev gene codes for a receptor protein, it turns out that the boss gene codes for a protein of a complementary type, called a signalling protein. Signalling proteins are a general class of molecule that match the shape of receptors, much as scents match the shape of receptor proteins in the nose. The difference is that whereas scents come from the outside, signalling proteins involved in development are produced by cells within the organism. Nevertheless, it will be helpful to use the notion of scents as a metaphor for such signalling proteins, so long as we remember that they are derived internally. This is similar to the way I have been using hidden colours as a metaphor for master proteins. We can summarise by saying that the boss gene produces a particular scent, the boss-signalling protein; whereas the sev gene produces a particular receptor, the sev-receptor protein.
Now there is a partnership between boss and sev, bemus e the boss-signalling protein matches the sev-receptor protein. In other words, the boss-signalling protein is the molecule, or internal scent, that the sev-receptor protein is sensitive to.
To see how this partnership between boss and sev influences eye development, look at Fig. 11.5, which shows two cells in the ommatidium of a normal fly: one that will become R7, and a neighbour that will become another type of cell, called R8 (their relative positions in the eye are shown in Fig. 11.3). First look at what happens in the cell on the left that will form R8. In this cell, the boss gene is switched on, and it therefore produces the boss-signalling protein (scent). The signalling protein sits in the cell membrane, with part of it exposed to the outside of the cell. Now look at the cell on the right. In this cell, the sev gene is switched on, so the sev-receptor protein
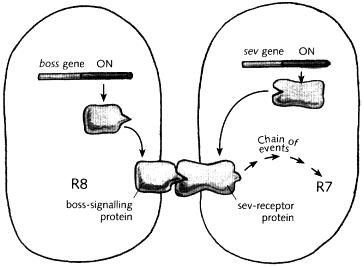
gets made. This protein moves to the cell membrane, where it then makes contact with the boss-signalling protein in the adjacent cell. The shape of the boss-signalling protein matches the sev-receptor and this sets off a chain of events in the responding cell, leading to its developing as R7 It is as if the responding cell is sniffing its neighbour, and when it detects a particular scent— the boss-signalling protein— it responds by becoming an R7 .
Fig. 11.5 Relationship between scents, sensitivities and cell fate in a fruit fly ommatidium The cell on the left (destined to become R8) produces a signalling protein from the boss gene, which triggers a receptor protein (produced by the sev gene) in the adjacent cell This sets off a chain of events which eventually lead to the cell acquiring an R7 identity
The partnership between sev and boss is a dog-like marriage, based on a chemical match between two partners (hence the name bride-of-sevenless). In mutants with a defective sev gene, the marriage breaks down because no sev-receptor protein is produced, rendering the responding cell blind to the scent In mutants defective for boss, no signalling protein is made, so there is no scent to stimulate the receptor
In many ways, the conclusions from these studies are similar to those of Spemann and Mangold with newt transplantations, In both cases, cells communicate with each other, influencing the way they develop The difference is that in the case of the eye, it was possible to identify the signalling molecules and the way they were responded to (the signalling molecules thought to underlie Spemann and Mangold's experiment have now been identified as well).
Oranges and lemons
I have given an example of how cells within the same organism can influence each other through molecular signals and receptors But in describing this case .
I had to postulate that there was already a difference between the two cells involved: in one cell the boss gene was switched on, whereas in the other cell the sev gene was switched on. If the two cells are already different to begin with, what does this chemical communication between them achieve? We shall see that this process can help to elaborate patterns of hidden colour during development. To explain how this happens, I shall begin with a hypothetical case so as to get some of the key parts of the message across, and then return to the case of the fruit fly's eye.
To see how cellular sniffing enables patterns to be refined, we will need to provide some
connections between scents, receptors and hidden colours. To get us going, I will make three rules that relate the hidden colours of a cell to its scents and sensitivities. The rules may seem rather arbitrary at first, but their molecular basis will become clear later on. Here are the three rules:
(1) A cell with a particular hidden colour gives off an associated scent. We can get an intuitive grasp of this rule because we commonly make associations between smells and colours. Orange, lemon, plum and strawberry are all colours, but they are also associated with particular scents: the smells of oranges, lemons, plums and strawberries. It is not that the colour pigments in the fruits are directly responsible for the smell, but the two are associated with each other. An orange fruit is both coloured orange, due to its pigment molecules, and gives off a particular scent, because it produces other molecules that can enter the air and be detected by our nose. In the same way, each hidden colour is not directly responsible for the scent given off by a cell, but is associated with it. Each hidden colour is a master protein of a particular type, whereas each scent is a signalling protein of a particular type.
(2) Each hidden colour is associated with a sensitivity to a particular scent. This is slightly more tricky to grasp than the previous rule. You have to imagine that if a cell has a hidden colour it will also respond to a particular type of scent. For example, a cell with a lemon hidden colour might respond to the scent of oranges. That is to say, it produces a receptor protein that matches the scent given off by an orange cell. Similarly, the orange cell might produce a receptor that is triggered by another scent, say the odour of plums. In this way each hidden colour is associated with a specific receptivity.
(3) When a cell detects a scent, it responds by changing its hidden colour. If a lemon cell should detect the scent of oranges, it will respond by changing to a new hidden colour, say from lemon to plum. Similarly, an orange cell that detects the smell of plums will react by changing to a different colour, say from orange to strawberry. It immediately follows from the first two rules that the new hidden colour will lead to a new scent (rule 1) and a new sensitivity (rule 2). The particular colour a cell changes to may vary according to the hidden colour it started off with and the type of scent it is exposed to. Hidden colours can change in response to the local scents, much as a chameleon might change its visible colour in response to its environment.
I now want to use these three rules to elaborate the pattern of hidden colours in a row of four cells. We shall start with two of the cells having one hidden colour, orange, and the other pair having a different hidden colour, lemon (Fig. 11.6). This initial arrangement will be built upon using our three rules to make a more elaborate pattern.
