
Solid-Phase Synthesis and Combinatorial Technologies
.pdf
7.4 ENCODING METHODS FOR SOLID-PHASE POOL LIBRARIES 313
|
NO2 |
|
OMe |
|
R1 |
|
|
|
N |
|
OMe |
|
R1 |
= |
Me |
HOOC |
Boc |
|
OMe |
M1 |
|
||
|
|
||
|
|
OMe |
|
|
7 representatives |
|
|
|
encoded by tags T1-T3 |
|
O |
HOOC
O
O
R2 = H (m-substituted acid chain in respect to the acetyl)
H (p-substituted)
Me (p-substituted)
OH
R2 M2a
3 representatives
encoded by tags T4,T5
HOOC
R2 = H (m-substituted acid chain in respect to the acetyl)
|
|
|
|
H (p-substituted) |
|
O |
Me (p-substituted) |
||
|
|
|||
O |
|
|
|
|
|
OH |
|
||
|
|
|||
|
|
|
||
R2 M |
|
|||
|
|
2b |
|
|
3 representatives |
|
|||
encoded by tags T6,T7
O |
O |
O |
Me |
|
|
|
N |
|
|
|
|||
|
|
|
O |
O |
O |
|
R3 |
R4 |
|
|
|||
|
|
|
|
|
||
M3a |
|
O |
OMe |
O |
|
|
7 representatives |
|
|
|
|||
|
NEt2 |
|
O |
S |
||
encoded by tags T8-T10 |
|
|
||||
|
|
|
|
|
||
Figure 7.33 Monomer structures and tags for the SP benzopyran libraries L6–L9: M1–M3a.
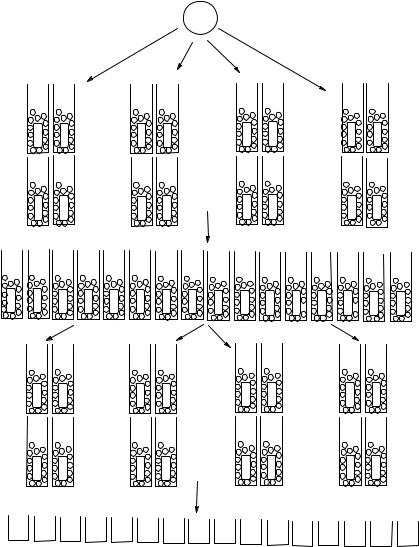
and coupled with the second monomer set (steps i–l). Finally the vessels are sorted according to the individual structures and placed in a cleavage plate (step m) where pure, released library individuals are recovered after the cleavage (step n). The preparation of the same 16-member library using parallel synthesis would have required 4 (coupling with monomers A–D) + 4 (deprotections) + 16 (coupling with monomers E–H) + 16 (cleavage) = 40 reactions. Directed sorting of radiofrequencyencoded vessels required 4 (coupling with monomers A–D) + 1 (deprotection) + 4

314 SYNTHETIC ORGANIC LIBRARIES: SOLID-PHASE POOL LIBRARIES
|
|
O |
|
O |
O |
|
O |
|
|
|
|
|
|
|
|||
|
R5 |
|
R6 |
|
|
O |
|
|
|
|
|
NBoc |
|
|
N |
|
|
|
|
M3b |
N |
|
|
|
|
|
|
3 representatives |
boc |
NH |
N |
|
|
||
encoded by tags T11,T12 |
boc |
|
|
|||||
|
boc |
|
|
|||||
|
|
|
|
|
|
|
|
|
M4 |
|
|
|
|
H2NO2S |
|
|
|
|
|
|
|
|
|
|
|
|
7 representatives |
|
|
|
COCl |
|
|
CHO |
|
acylating/alkylating |
|
SO2Cl |
|
COOH |
||||
|
N |
|
|
|
||||
agents |
|
|
|
|
N |
|
||
encoded by tags T13-T15 |
CHO |
|
|
|
||||
N Cl |
|
O |
|
|||||
|
|
|
|
|
|
|
||
NH2 |
|
|
Me |
|
OMe |
|
|
|
|
|
|
|
|
|
|
||
R8 |
|
|
|
|
|
|
|
|
M5 |
|
|
|
|
|
|
|
|
7 representatives |
|
|
|
|
|
|
|
|
encoded by tags T16-T18 |
|
|
|
|
|
|
||
|
|
|
NHBoc |
|
NHFmoc |
|
NH |
|
M6 |
HOOC |
|
NHBoc |
HOOC |
N |
NHPMC |
|
|
|
|
|
|
|
H |
|
|
|
10 representatives |
|
|
N |
N |
|
N |
|
|
acylating |
|
N |
|
|
|
|||
|
|
|
|
O |
|
|
||
|
|
|
S |
|
S |
|
||
agents |
|
|
O |
|
|
|||
|
|
|
|
|
|
|
||
spatially |
|
|
|
|
|
|
|
|
encoded |
|
|
|
|
|
COOH |
SO2Cl |
|
|
|
|
|
|
|
|
||
|
|
|
COCl |
|
|
|
|
|
|
|
|
COCl |
|
Me |
|
||
|
|
|
O |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
Figure 7.34 Monomer structures and tags for the SP benzopyran libraries L6–L9: M3b–M6.
(coupling with monomers E–H) + 16 (cleavage) = 25 reactions. This reduction in the number of reactions performed becomes more significant when the number of chemical steps or the number of monomers increase and allows an easier and faster SPS of discretes using a modified mix-and-split technique. The apparatus to perform radio- frequency-encoded SP reactions is commercially available using either resin beads in microvessels (227), grafted microtubes (228, 229) or pins as reactors (230), and the SPS of libraries with up to several thousands of individuals with good yields and purities have been reported (231–235). The interest generated by this technique has recently stimulated improvements in the handling and sorting of large amounts of microreactors (236), in the final cleavage protocols (237), and in their direct use for

|
7.4 ENCODING METHODS FOR SOLID-PHASE POOL LIBRARIES |
315 |
|||||
|
O |
|
|
OH |
|
|
|
|
O |
NH |
|
|
|
|
|
O NH |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
O |
O |
|
O |
O |
N O |
|
|
O |
|
Me |
|
|
|||
|
N |
|
|
|
|
||
|
Me |
|
|
7.56(L7) |
NH |
|
|
|
7.55(L6) |
NH |
|
|
|
||
|
|
|
|
|
|
||
OMe |
|
|
OMe |
|
|
|
|
MeO |
O |
MeO |
|
O |
Me |
|
|
|
|
|
Me |
|
|||
|
|
|
|
|
O |
|
|
|
N |
|
|
N |
O |
|
|
|
|
|
|
Me |
|||
|
H |
|
|
H |
|
||
|
7.57(L6) |
|
O |
7.58(L9) |
|
|
|
|
O |
|
N |
|
|||
|
|
|
|
|
|
||
|
|
|
|
|
|
SO2Me |
|
|
|
|
O |
|
|
|
|
OMe |
|
|
|
OMe |
|
|
|
MeO |
O |
Me |
MeO |
|
O |
Me |
|
|
|
|
Me |
||||
|
O |
Me |
|
|
O |
O |
|
|
O |
|
|
|
|||
|
N |
|
Me |
|
N |
|
Me |
|
H |
|
|
H |
|
||
|
7.59(L6) |
O |
|
|
7.60(L8) |
S |
S |
|
|
|
|
|
|
|
|
Figure 7.35 Structures of six characterized individuals 7.55–7.60 from L6–L9.
biological screening (238). We can easily foresee a steady and copious flow of reports of radiofrequency-encoded SP libraries in the future, especially considering the reasonable cost of the necessary equipment, which is already present in most laboratories that perform combinatorial synthesis of chemical libraries.
7.4.4 An Example: Synthesis of a Tyrphostin Radiofrequence Encoded
Library
Recently Shi et al. (239) reported the synthesis of a 432-member focused library L10 as a source of tyrosine kinase inhibitors using benzylidene malononitriles, or tyrphostins, as structural motifs to design the library. Both the general tyrphostin structure (7.61) and an example of an active compound on a specific tyrosine kinase (7.62) are reported in Fig. 7.37.
The reactors selected were 432 aminomethylated MicroTubes 7.63, which are polystyrene-grafted polypropylene tubes with a ≈50 M loading capacity per reactor, that encapsulate a preencoded radiofrequency tag (Fig. 7.38). The tubes were reacted with the Fmoc-protected, acid-labile Knorr linker (step a), the residual amine functions
316 SYNTHETIC ORGANIC LIBRARIES: SOLID-PHASE POOL LIBRARIES
|
|
a,b |
|
a,c |
|
|
a,e |
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
a,d |
|
|
|
|
|
|
A |
A |
B |
B |
|
C |
C |
D |
D |
|
|
E |
F |
E |
F |
|
E |
F |
E |
F |
|
|
A |
A |
B |
B |
|
C |
C |
D |
D |
|
|
G |
H |
G |
H |
|
G |
H |
G |
H |
|
|
|
|
|
|
f,g |
|
|
|
|
|
A A |
A A B B |
B B C C |
C C D D |
D |
D |
|||||
E |
F |
G H E |
F |
G H E |
F |
G H E |
F |
G |
H |
|
|
h,i |
|
|
h,j |
h,k |
|
|
h,l |
|
|
|
A |
B |
A |
B |
|
A |
B |
A |
B |
|
|
E |
E |
F |
F |
|
G |
G |
H |
H |
|
|
C |
D |
C |
D |
|
C |
D |
C |
D |
|
|
E |
E |
F |
F |
|
G |
G |
H |
H |
|
|
|
|
|
|
m,n |
|
|
|
|
|
AE |
AF |
AG AH BE |
BF |
BG |
BH CE |
CF |
CG CH DE |
DF |
DG |
DH |
16-member discrete library of released compounds
a: sorting according to first monomer addition; b-e, i-l: coupling of resin with monomer: A (b), B (c), C (d), D (e), E (i), F (j), G (k), H (l); f: mix in one pool; g: deprotection; h: sorting according to second monomer addition; m: sorting according to individual structures; n: cleavage.
Figure 7.36 Radiofrequency encoding: directed sorting for a hypothetical 16-member discrete library.
were capped (step b), and the Fmoc group was removed (step c) to give 7.64 with a ≈50 M loading (Fmoc reading). The above reactions were performed in a single flask, while the coupling with M1 (18 aromatic aldehydes) required prior sorting of the preencoded tubes (step d) and their partitioning into 18 flasks, where each aldehyde

7.4 ENCODING METHODS FOR SOLID-PHASE POOL LIBRARIES 317 |
|||
R2 |
CN |
HO |
CN |
|
|
||
|
|
H |
|
|
|
|
|
|
R1 |
HO |
N |
(R3O)n |
|
||
|
|
O |
|
7.61 |
O |
7.62 |
|
|
|
|
|
Figure 7.37 General structure of tyrphostins (7.61) and of an active compound on a tyrosine kinase (7.62).
was coupled to 24 tubes (step e), and the corresponding imines were reduced (step f) to give secondary amines 7.65. All the reactors were then pooled (step g) and coupled with cyanoacetic acid using multiple cycles (step h) to give the amides 7.66. The tubes were then sorted according to M2 (eight aromatic aldehydes bearing a phenol group,
RFTag
|
|
|
|
|
|
|
CHO |
|
|
|
|
|
|
NH2 |
a-d |
H |
NH2 |
|
|
e-g |
H |
H |
|
|
|
|
|
N |
+ |
|
N |
N |
|
|
||||
|
|
|
L |
|
|
|
|
L |
|
|
R1 |
|
|
7.63 |
|
7.64 |
|
|
R1 |
|
|
7.65 |
|
|
|
|
|
|
|
M1 |
|
|
|
|
|
|||
PS-grafted |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
tubes |
|
18 sorted pools |
|
18 representatives |
|
|
|
|
||||
|
|
|
24 tubes/pool |
|
|
|
|
|
|
|
|
|
|
CN |
|
|
|
CHO |
|
(OH)n |
|
|
|
||
|
|
|
O |
|
|
R2 |
|
CN |
|
|
||
|
H |
|
|
|
|
|
|
|
O |
|
|
|
h,i |
N |
|
+ |
|
|
|
j,k |
|
|
|
||
|
|
|
|
|
|
|
|
|||||
N |
|
|
|
(OH)n |
|
H |
|
|
+ |
|||
|
L |
|
R1 |
|
|
|
N |
|
||||
|
|
R2 |
|
|
|
N |
|
|
||||
|
7.66 |
|
|
|
|
|
L |
|
R |
|||
|
|
|
|
M2 |
|
|
7.67 |
|
1 |
|||
|
8 sorted pools |
|
|
|
|
|
|
|||||
|
54 tubes/pool |
8 representatives |
|
3 sorted pools |
|
|||||||
|
|
|
|
|
|
|
|
|
144 tubes/pool |
|
||
|
|
|
(OCOR3)n |
|
|
|
(OCOR3)n |
|
||||
|
|
|
|
|
|
CN |
|
|||||
|
|
|
|
|
CN |
|
|
R2 |
|
|||
|
|
|
R2 |
|
|
|
|
|
O |
|||
|
COCl |
|
|
|
|
O |
|
|
|
|||
+ R |
l |
|
|
|
|
|
|
|
||||
|
|
|
|
|
m,n |
|
|
|
||||
3 |
M3 |
|
|
H |
|
|
|
|
L10 |
NH |
||
|
|
|
|
N |
|
|
||||||
|
|
|
N |
|
|
|
|
R1 |
||||
3 representatives |
|
|
|
L |
|
R1 |
432 discretes |
|
||||
7.68
a: Knorr linker, PyBOP, DIPEA, DCM; b: Ac2O, DIPEA; c: 20% piperidine/DMF; d: sorting according to M1; e: coupling with M1; f: NaCNBH3, AcOH; g: mix in one pool; h: cyanoacetic acid,
DIC, DMF, three cycles; i: sorting according to M2; j: coupling with M2; k: sorting according to M3; l: coupling with M3; m: sorting as individual compounds; n: 4% TFA/benzene.
L = Knorr linker
Figure 7.38 SP synthesis of a radiofrequency encoded, 432-member discrete tyrphostininspired library L10.
318 SYNTHETIC ORGANIC LIBRARIES: SOLID-PHASE POOL LIBRARIES
step i), and the aldol condensation was performed in eight flasks, each containing 54 tubes (step j), to give the unsaturated nitriles 7.67. Finally, the tubes were again sorted, this time according to M3 (two acyl chlorides and an empty position to keep the free phenol, step k), and coupled to give the final supported esters/phenols 7.68 (step l). These were sorted and identified (step m), then accordingly arranged in 432 positions of several microplates and cleaved (step n, Fig. 7.38) to give the library L10 as 432 discretes with good yields and purities determined by TLC of all samples and by MS and NMR of 5% of library compounds selected randomly (24 samples).
The power of this encoding method to prepare discrete libraries is again summarized by the number of chemical reactions performed. If the library had been prepared by parallel synthesis, a total of 1 (step a) + 1 (step b) + 1 (step c) + 18 (step e) + 18 (step f) + 18 (step h) + 144 (step j) + 288 (step l) + 432 (step n) = 921 reactions would have been necessary. Directed sorting allowed the number to be limited to 1 (step a) + 1 (step b) + 1 (step c) + 18 (step e) + 18 (step f) + 1 (step h) + 8 (step j) + 2 (step l) + 432 (step n) = 482 reactions, while ensuring the same quality of the final discrete products.
7.5 NEW TRENDS IN SOLID-PHASE POOL LIBRARIES
7.5.1 Bead-Based Libraries: High-Throughput Synthesis
Many factors have recently contributed to a general decrease of interest for large, primary SP pool libraries. These include the increased throughput of solution and SP discrete libraries, the advent of computational tools to create virtual libraries and to rationally select smaller subsets to be prepared, and the assumed lower quality of large SP pool libraries, which originates from their more difficult analytical characterization. Despite all these factors, some recent examples of bead-based pool libraries have clearly shown how a rigorous, integrated approach may produce high-quality, very large primary or biased-targeted libraries to be tested using HTS. The amount of information generated by these libraries, together with the moderate amount of efforts necessary, should convince even the more conservative chemist of the huge potential embedded into this library format and that a rigorous chemical approach is the gateway to successful SP pool libraries.
We will present here a very recent example by Tan et al. (196, 240), who are heavily involved in the so-called chemical genetic approach, (see Section 9.1.4) where large numbers of compounds must be routinely screened to identify small-molecule ligands/inhibitors, which are able to influence as many gene products as possible. This group developed some miniaturized, cell-based HTS (241–244), which allow testing of bead-based libraries and detection of active, cell-permeable compounds that interact with proteins (242), or even compounds that influence posttranslational modifications of gene products (243). These miniaturized assays require large collections of meaningful small molecules to be tested, and the authors decided to use encoded (189), bead-based libraries prepared by mix-and-split methods. They introduced some limitations upfront, such as water-compatible Tentagel supports and a photolabile linker
7.5 NEW TRENDS IN SOLID-PHASE POOL LIBRARIES 319
(245) which are limiting the choice among many organic reaction conditions, and also decided to start from structures similar to known natural products and to combinatorialize them to obtain millions of small organic compounds. Even more importantly, they decided to be extremely rigorous in assessing the purity of such a large SP pool library.
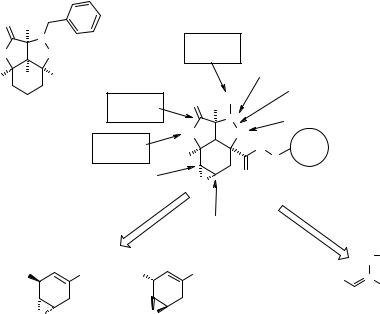
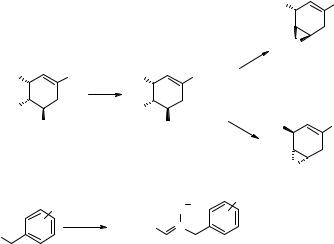
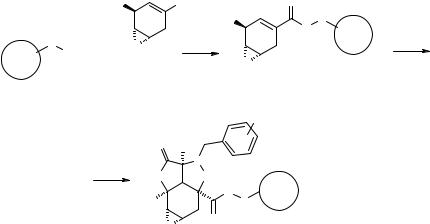
The authors chose as a template to inspire their large bead-based library synthesis the compound 7.69 (Fig. 7.39) (246, 247). This polycyclic scaffold is interesting as such, representing a constrained, highly functionalized small organic molecule, but it can also be considered as a highly flexible, geometrically and stereochemically pure scaffold where to introduce chemical diversity by mild chemical transformations without the need of protection/deprotection steps. A reasonable retrosynthetic scheme was designed to provide the 7.69-inspired SP scaffold 7.70 (Fig. 7.39). This scaffold allows a priori four primary diversifications (Fig. 7.39, full arrows): 1) nucleophilic addition to the epoxide, 2) nucleophilic addition to the lactone, 3) decoration of the R1 substituent (either through different nitrones, or using functionalizable nitrones), and 4) reductive cleavage of the N-O isoxazoline bond. Transformations 1, 2 and 4, if successfully assessed, would open secondary diversifications (Fig. 7.39, dashed arrows): 1′) decoration of the hydroxyl function originated from epoxide opening; 2′) decoration of the hydroxyl function originated from lactone opening; 3′) decoration
O |
H |
|
|
|
|
|
|
N |
|
|
nitrone |
|
|
O |
|
O |
|
decoration |
amine |
|
|
|
|
|
|
|
decoration reductive |
H |
H |
H |
|
|
|
N-O bond |
|
|
|
|
|
|
cleavage |
|
7.69 |
|
lactone |
O H |
R1 |
alcohol |
|
|
|
opening |
|
N |
decoration |
|
|
|
|
|
||
|
|
alcohol |
O |
O |
||
|
|
|
|
H |
||
|
|
decoration |
H |
|
N |
|
|
|
|
|
|
L |
|
|
|
|
alcohol |
O |
|
O |
|
|
decoration |
|
7.70 |
||
|
|
|
|
|
|
|
|
|
|
|
nucleophilic |
||
|
|
|
|
epoxide |
|
|
|
|
|
|
opening |
|
|
|
|
|
HO |
COOH |
|
O |
|
HO |
COOH |
|
HOOC N |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
R1 |
|
|
|
|
|
|
7.72 |
|
O |
|
O |
|
|
|
|
(+)-7.71 |
(-)-7.71 |
|
|
||
Figure 7.39 Bead-based libraries: structures of a natural product-biased scaffold 7.69 and of its adapted SP version 7.70 for the synthesis of a large bead-based library.
322 SYNTHETIC ORGANIC LIBRARIES: SOLID-PHASE POOL LIBRARIES
|
|
|
|
|
|
|
|
|
|
|
|
|
R1 |
|
|
|
I |
|
|
|
|
|
|
|
|
|
|
|
|
O |
H |
|
|
|
|
|
O |
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
N |
|
|
|
|
|
|
N |
|
|
|
|
|
|
O |
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
O |
|
O |
H |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
N |
L |
|
|
R1 a |
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
N L |
|
|
|||||
|
S |
|
|
+ |
H |
|
|
|
|
|||||
|
O |
|
|
|
|
|
|
|
|
S |
|
|
||
|
|
|
|
|
|
|
|
O 7.76a-c |
|
|
||||
O |
|
|
|
|
|
O |
|
|
||||||
|
|
|
|
|
|
|
|
7.77a-c |
|
|
||||
|
L = photolinker |
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
S = absent (7.74a-c) |
|
|
|
|
+ |
|
|
NH2 |
|
|
|||
|
S = spacer (7.75a-c) |
|
|
R1 |
|
R |
|
|
R1 |
|||||
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
2 |
|
|
|
||
|
R2 NH |
|
|
|
|
|
R2 |
NH |
|
|
|
|
|
|
|
O |
|
N |
|
+ |
COOH |
|
O |
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
b |
|
O |
H |
R3 |
R3 |
O |
|
|
|
O |
H |
|
|
|
HO |
|
|
L |
|
|
|
|
|
L |
||||
|
|
|
|
N |
c |
|
|
|
|
|
|
N |
||
|
|
|
|
|
S |
|
|
|
|
|
|
S |
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
||
|
|
|
O |
|
|
|
|
|
|
|
O |
|
|
|
|
|
O |
|
|
|
|
|
O |
|
|
7.80a-c |
|||
|
|
|
7.78a-c |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
7.79a-c |
|
|
|
|
|
|
|
7.81a-c |
||
|
|
|
|
|
|
EtO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OEt |
|
|
|
|
|
|
|
|
|
OMe |
OMe |
|
|
|
|
|
|
|
|
|
|
|
|
d |
|
|
NH |
|
|
|
|
NH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
N |
O |
and |
|
O |
N |
O |
|
|
|
O |
|
|
O |
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
O |
|
|
H |
|
||||
|
|
|
|
NH2 |
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
N |
|
NH2 |
||
|
|
O |
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
O |
7.83b |
|
||||
|
7.82b |
O |
|
|
|
O |
|
|
||||||
|
|
|
|
|
|
|
|
|
||||||
7.81a R1 = t-Bu (o-substituted); R2 = p-MeOBenzyl; R3 = i-Pr 7.81c R1 = n-Pr (p-substituted); R2 = p-MeOPhenethyl; R3 = Et
7.83a R1 = benzyl (o-substituted); R2 = cyclobutyl; R3 = p-MeOBenzyl
7.83c R1 = p-ClPhenyl (p-substituted); R2 = o-MeOBenzyl; R3 = i-Pr
a:CuI, (PPh3)2PdCl2, DIPEA, DMF, rt, 15-45'; b: 2-HOPyridine, THF, rt, 12-16 hrs;
c:DIC, DIPEA, DMAP, DCM, rt, 12-16 hrs; d: cleavage.
Figure 7.42 SP chemistry assessment for the large, natural products–biased library L12: compounds 7.82a–c and 7.83a–c.
second monomer set of 62 rehearsed primary amines. A similar procedure was repeated with resin-bound 7.78a, obtained by reaction of 7.76a with p-methoxybenzyl amine, and 98 carboxylic acids were tested to eventually produce a third rehearsed monomer set made of 62 carboxylic acids (44 high performing, 18 less than optimal, Fig. 7.43).
