- •Introduction
- •Introduction
- •Important types of plastics
- •Incineration
- •Introduction
- •In this equation, f is the force of friction, µ is the coefficient of friction between the object and the surface, and n is the normal force.
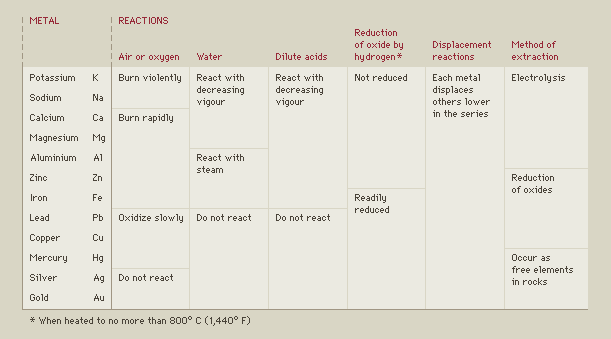
- •Reactivity Series
- •Metallic Bonding
- •Lathe, Milling Machine, Planer, and Shaper
- •Some Conventional Machine Tools
In this equation, f is the force of friction, µ is the coefficient of friction between the object and the surface, and n is the normal force.
Scientists have measured the coefficient of friction for many combinations of materials. Coefficients of friction depend on whether the objects are initially moving or stationary and on the types of material involved. The coefficient of friction for rubber sliding on concrete is 0.8 (relatively high), while the coefficient for Teflon sliding on steel is 0.04 (relatively low).
The normal force is the force the object exerts perpendicular to the surface. In the case of a level surface, the normal force is equal to the weight of the object. If the surface is inclined, only a fraction of the object’s weight pushes directly into the surface, so the normal force is less than the object’s weight.
KINDS OF FRICTION
Different kinds of motion give rise to different types of friction between objects. Static friction occurs between stationary objects, while sliding friction occurs between objects as they slide against each other. Other types of friction include rolling friction and fluid friction. The coefficient of friction for two materials may differ depending on the type of friction involved.
Static friction prevents an object from moving against a surface. It is the force that keeps a book from sliding off a desk, even when the desk is slightly tilted, and that allows you to pick up an object without the object slipping through your fingers. In order to move something, you must first overcome the force of static friction between the object and the surface on which it is resting. This force depends on the coefficient of static friction (µs) between the object and the surface and the normal force (N) of the object.
A book sliding off a desk or brakes slowing down a wheel are both examples of sliding friction, also called kinetic friction. Sliding friction acts in the direction opposite the direction of motion. It prevents the book or wheel from moving as fast as it would without friction. When sliding friction is acting, another force must be present to keep an object moving. In the case of a book sliding off a desk, this force is gravity. The force of kinetic friction depends on the coefficient of kinetic friction between the object and the surface on which it is moving (µk) and the normal force (N) of the object. For any pair of objects, the coefficient of kinetic friction is usually less than the coefficient of static friction. This means that it takes more force to start a book sliding than it does to keep the book sliding.
Rolling friction hinders the motion of an object rolling along a surface. Rolling friction slows down a ball rolling on a basketball court or softball field, and it slows down the motion of a tire rolling along the ground. Another force must be present to keep an object rolling. For example, a pedaling bicyclist provides the force necessary to the keep a bike in motion. Rolling friction depends on the coefficient of rolling friction between the two materials (µr) and the normal force (N) of the object. The coefficient of rolling friction is usually about that of sliding friction. Wheels and other round objects will roll along the ground much more easily than they will slide along it.
Objects moving through a fluid experience fluid friction, or drag. Drag acts between the object and the fluid and hinders the motion of the object. The force of drag depends upon the object’s shape, material, and speed, as well as the fluid’s viscosity. Viscosity is a measure of a fluid’s resistance to flow. It results from the friction that occurs between the fluid’s molecules, and it differs depending on the type of fluid. Drag slows down airplanes flying through the air and fish swimming through water. An airplane’s engines help it overcome drag and travel forward, while a fish uses its muscles to overcome drag and swim. Calculating the force of drag is much more complicated than calculating other types of friction.
EFFECTS OF FRICTION
Friction helps people convert one form of motion into another. For example, when people walk, friction allows them to convert a push backward along the ground into forward motion. Similarly, when car or bicycle tires push backward along the ground, friction with the ground makes the tires roll forward. Friction allows us to push and slide objects along the ground without our shoes slipping along the ground in the opposite direction.
While friction allows us to convert one form of motion to another, it also converts some energy into heat, noise, and wear and tear on material. Losing energy to these effects often reduces the efficiency of a machine. For example, a cyclist uses friction between shoes and pedals, the chain and gears, and the bicycle’s tires and the road to make the bicycle move forward. At the same time, friction between the chain and gears, between the tires and the road, and between the cyclist and the air all resist the cyclist’s motion. As the cyclist pedals, friction converts some of the cyclist’s energy into heat, noise, and wear and tear on the bicycle. This energy loss reduces the efficiency of the bicycle. In automobiles and airplanes, friction converts some of the energy in the fuel into heat, noise, and wear and tear on the engine’s parts. Excess frictional heat can damage an engine and braking system. The wearing away of material in engines makes it necessary to periodically replace some parts.
Sometimes the heat that friction produces is useful. When a person strikes a match against a rough surface, friction produces a large amount of heat on the head of the match and triggers the chemical process of burning. Static friction, which prevents motion, does not create heat.
REDUCING FRICTION
Reducing the amount of friction in a machine increases the machine’s efficiency. Less friction means less energy lost to heat, noise, and wearing down of material. People normally use two methods to reduce friction. The first method involves reducing the roughness of the surfaces in contact. For example, sanding two pieces of wood lessens the amount of friction that occurs between them when they slide against one another. Teflon creates very little friction because it is so smooth.
Applying a lubricant to a surface can also reduce friction. Common examples of lubricants are oil and grease. They reduce friction by minimizing the contact between rough surfaces. The lubricant’s particles slide easily against each other and cause far less friction than would occur between the surfaces. Lubricants such as machine oil reduce the amount of energy lost to frictional heating and reduce the wear damage to the machine surfaces caused by friction.
Materials Science and Technology
|
I |
|
INTRODUCTION |
Materials Science and Technology, the study of materials, nonmetallic as well as metallic, and how they can be adapted and fabricated to meet the needs of modern technology. Using the laboratory techniques and research tools of physics, chemistry, and metallurgy, scientists are finding new ways of using plastics, ceramics, and other nonmetals in applications formerly reserved for metals.
|
II |
|
RECENT DEVELOPMENTS |
The rapid development of semiconductors (see Semiconductor) for the electronics industry, beginning in the early 1960s, gave materials science its first major impetus. Having discovered that nonmetallic materials such as silicon could be made to conduct electricity in ways that metals could not, scientists and engineers devised ways of fashioning thousands of tiny integrated circuits (see Integrated Circuit) on a small chip of silicon. This then made it possible to miniaturize the components of electronic devices such as computers.
In the late 1980s, materials science research was given renewed emphasis with the discovery of ceramics that display superconductivity at higher temperatures than metals do. If the temperature at which these new materials become superconductive can be raised high enough, new applications, including levitating trains and superfast computers, are possible.
Although the latest developments in materials science have tended to focus on electrical properties, mechanical properties are also of major, continuing importance. For the aircraft industry, for instance, scientists have been developing, and engineers testing, nonmetallic composite materials that are lighter, stronger, and easier to fabricate than the aluminum and other metals currently used to form the outer skin of aircraft.
|
III |
|
MECHANICAL PROPERTIES OF MATERIALS |
Engineers must know how solid materials respond to external forces, such as tension, compression, torsion, bending, and shear. Solid materials respond to these forces by elastic deformation (that is, the material returns to its original size and form when the external force is lifted), permanent deformation, or fracture. Time-dependent effects of external forces are creep and fatigue, which are defined below.
Tension is a pulling force that acts in one direction; an example is the force in a cable holding a weight. Under tension, a material usually stretches, returning to its original length if the force does not exceed the material's elastic limit (see Elasticity). Under larger tensions, the material does not return completely to its original condition, and under even greater forces the material ruptures.
Compression is the decrease in volume that results from the application of pressure. When a material is subjected to a bending, shearing, or torsional (twisting) force, both tensile and compressive forces are simultaneously at work. When a rod is bent, for example, one side of it is stretched and subjected to a tensional force, and the other side is compressed.
Creep is a slowly progressing, permanent deformation that results from a steady force acting on a material. Materials subjected to high temperatures are especially susceptible to this deformation. The gradual loosening of bolts, the sagging of long-span cables, and the deformation of components of machines and engines are all noticeable examples of creep. In many cases the slow deformation stops because the force causing the creep is eliminated by the deformation itself. Creep extended over a long time eventually leads to the rupture of the material.
Fatigue can be defined as progressive fracture. It occurs when a mechanical part is subjected to a repeated or cyclic stress, such as vibration. Even when the maximum stress never exceeds the elastic limit, failure of the material can occur even after a short time. With some metals, such as titanium alloys, fatigue can be avoided by keeping the cyclic force below a certain level. No deformation is apparent during fatigue, but small localized cracks develop and propagate through the material until the remaining cross-sectional area cannot support the maximum stress of the cyclic force. Knowledge of tensile stress, elastic limits, and the resistance of materials to creep and fatigue are of basic importance in engineering. See also Metals.
Superconductivity
|
I |
|
INTRODUCTION |
Superconductivity, phenomenon displayed by certain conductors that demonstrate no resistance to the flow of an electric current. Superconductors also exhibit strong diamagnetism; that is, they are repelled by magnetic fields. Superconductivity is manifested only below a certain critical temperature Tc and a critical magnetic field Hc, which vary with the material used. Before 1986, the highest Tc was 23.2 K (-249.8° C/-417.6° F) in niobium-germanium compounds. Temperatures this low were achieved by use of liquid helium, an expensive, inefficient coolant. Ultralow-temperature operation places a severe constraint on the overall efficiency of a superconducting machine. Thus, large-scale operation of such machines was not considered practical. But in 1986 discoveries at several universities and research centers began to radically alter this situation. Ceramic metal-oxide compounds containing rare earth elements were found to be superconductive at temperatures high enough to permit using liquid nitrogen as a coolant. Because liquid nitrogen, at 77K (-196° C/-321° F), cools 20 times more effectively than liquid helium and is 10 times less expensive, a host of potential applications suddenly began to hold the promise of economic feasibility. In 1987 the composition of one of these superconducting compounds, with Tc of 94K (-179° C/-290° F), was revealed to be YBa2Cu307 (yttrium-barium-copper-oxide). It has since been shown that rare-earth elements, such as yttrium, are not an essential constituent, for in 1988 a thallium-barium-calcium copper oxide was discovered with a Tc of 125K (-148° C/-234° F).
|
II |
|
HISTORY |
Superconductivity was first discovered in 1911 by the Dutch physicist Heike Kamerlingh Onnes, who observed no electrical resistance in mercury below 4.2 K (-268.8° C/-451.8° F). The phenomenon was better understood only after strong diamagnetism was detected in a superconductor by Karl W. Meissner and R. Ochsenfeld of Germany in 1933. The basic physics of superconductivity, however, was not realized until 1957, when the American physicists John Bardeen, Leon N. Cooper, and John R. Schrieffer advanced the now celebrated BCS theory, for which the three were awarded the 1972 Nobel Prize in physics. The theory describes superconductivity as a quantum phenomenon (see Quantum Theory), in which the conduction electrons move in pairs and thus show no electrical resistance. In 1962 the British physicist Brian D. Josephson examined the quantum nature of superconductivity and proposed the existence of oscillations in the electric current flowing through two superconductors separated by a thin insulating layer in a magnetic or electric field. The effect, known as the Josephson effect, subsequently was confirmed by experiments.
|
III |
|
APPLICATIONS |
Because of their lack of resistance, superconductors have been used to make electromagnets that generate large magnetic fields with no energy loss. Superconducting magnets have been used in diagnostic medical equipment, studies of materials, and in the construction of powerful particle accelerators. Using the quantum effects of superconductivity, devices have been developed that measure electric current, voltage, and magnetic field with unprecedented sensitivity.
The discovery of better superconducting compounds is a significant step toward a far wider spectrum of applications, including faster computers with larger storage capacities, nuclear fusion reactors in which ionized gas is confined by magnetic fields, magnetic levitation (lifting or suspension) of high-speed (“Maglev”) trains, and perhaps most important of all, more efficient generation and transmission of electric power. The 1987 Nobel Prize in physics went to West German physicist J. Georg Bednorz and Swiss physicist K. Alex Müller for their discovery of materials that are superconductive at temperatures higher than had been thought possible. See Electricity; Magnetism.
Metals
|
I |
|
INTRODUCTION |
Metals, group of chemical elements that exhibit all or most of the following physical qualities: they are solid at ordinary temperatures; opaque, except in extremely thin films; good electrical and thermal conductors (see Conductor, Electrical); lustrous when polished; and have a crystalline structure when in the solid state. Metals and nonmetals are separated in the periodic table by a diagonal line of elements. Elements to the left of this diagonal are metals, and elements to the right are nonmetals. Elements that make up this diagonal—boron, silicon, germanium, arsenic, antimony, tellurium, polonium, and astatine—have both metallic and nonmetallic properties. The common metallic elements include the following: aluminum, barium, beryllium, bismuth, cadmium, calcium, cerium, chromium, cobalt, copper, gold, iridium, iron, lead, lithium, magnesium, manganese, mercury, molybdenum, nickel, osmium, palladium, platinum, potassium, radium, rhodium, silver, sodium, tantalum, thallium, thorium, tin, titanium, tungsten, uranium, vanadium, and zinc. Metallic elements can combine with one another and with certain other elements, either as compounds, as solutions, or as intimate mixtures. A substance composed of two or more metals, or a substance composed of a metal and certain nonmetals such as carbon are called alloys. Alloys of mercury with other metallic elements are known as amalgams.
Within the general limits of the definition of a metal, the properties of metals vary widely. Most metals are grayish in color, but bismuth is pinkish, copper is red, and gold is yellow. Some metals display more than one color, a phenomenon called pleochroism. The melting points of metals range from about -39° C (about -38° F) for mercury to 3410° C (6170° F) for tungsten. Osmium and iridium (specific gravity 22.6) are the most dense metals, and lithium (specific gravity 0.53) is the least dense. The majority of metals crystallize in the cubic system, but some crystallize in the hexagonal and tetragonal systems (see Crystal). Bismuth has the lowest electrical conductivity of the metallic elements, and silver the highest at ordinary temperatures. (For conductivity at low temperatures, see Cryogenics; Superconductivity.) The conductivity of most metals can be lowered by alloying. All metals expand when heated and contract when cooled, but certain alloys, such as platinum and iridium alloys, have extremely low coefficients of expansion.
|
II |
|
PHYSICAL PROPERTIES |
Metals are generally very strong and resistant to different types of stresses. Though there is considerable variation from one metal to the next, in general metals are marked by such properties as hardness, the resistance to surface deformation or abrasion; tensile strength, the resistance to breakage; elasticity, the ability to return to the original shape after deformation; malleability, the ability to be shaped by hammering; fatigue resistance, the ability to resist repeated stresses; and ductility, the ability to undergo deformation without breaking. See Materials Science and Technology.
|
III |
|
CHEMICAL PROPERTIES |