- •Introduction
- •Introduction
- •Important types of plastics
- •Incineration
- •Introduction
- •In this equation, f is the force of friction, µ is the coefficient of friction between the object and the surface, and n is the normal force.
- •Reactivity Series
- •Metallic Bonding
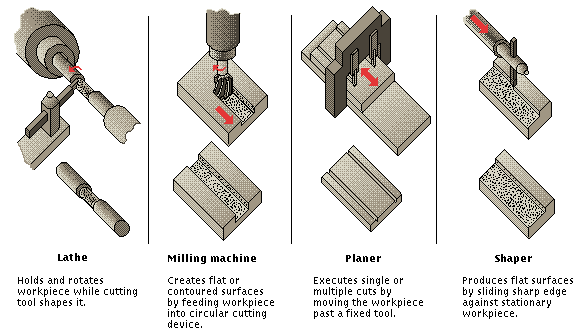
- •Lathe, Milling Machine, Planer, and Shaper
- •Some Conventional Machine Tools
Reactivity Series
Chemists can list metals according to how quickly they undergo chemical reactions, such as burning or dissolving in acids. The result is called a reactivity series. A metal at the top of the series generally reacts more vigorously than those that are below it in the series, and the more reactive metal can take their place (or displace them) in various compounds or in solution. In some reactions, however, such as reduction reactions, the order of reactivity is reversed.
Metals typically have positive valences in most of their compounds, which means they tend to donate electrons to the atoms to which they bond. Also, metals tend to form basic oxides. Typical nonmetallic elements, such as nitrogen, sulfur, and chlorine, have negative valences in most of their compounds—meaning they tend to accept electrons—and form acidic oxides (see Acids and Bases; Chemical Reaction).
Metals typically have low ionization potentials. This means that metals react easily by loss of electrons to form positive ions, or cations. Thus, metals can form salts (chlorides, sulfides, and carbonates, for example) by serving as reducing agents (electron donors).
|
IV |
|
ELECTRON STRUCTURE |
In early attempts to explain the electronic configurations of the metals, scientists cited the characteristics of high thermal and electrical conductivity in support of a theory that metals consist of ionized atoms in which the free electrons form a homogeneous sea of negative charge. The electrostatic attraction between the positive metal ions and the free-moving and homogeneous sea of electrons was thought to be responsible for the bonds between the metal atoms. Free movement of the electrons was then held to be responsible for the high thermal and electrical conductivities. The principal objection to this theory was that the metals should then have higher specific heats than they do.
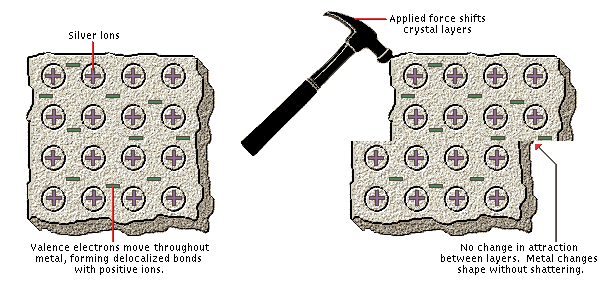
Metallic Bonding
Silver, a typical metal, consists of a regular array of silver atoms that have each lost an electron to form a silver ion. The negative electrons distribute themselves throughout the entire piece of metal and form nondirectional bonds between the positive silver ions. This arrangement, known as metallic bonding, accounts for the characteristic properties of metals: they are good electrical conductors because the electrons are free to move from one place to another, and they are malleable (as shown here) because the positive ions are held together by nondirectional forces.
In 1928 the German physicist Arnold Sommerfeld proposed that the electrons in metals exist in a quantized arrangement in which low energy levels available to the electrons are almost fully occupied (see Atom; Quantum Theory). In the same year the Swiss-American physicist Felix Bloch and later the French physicist Louis Brillouin used this idea of quantization in the currently accepted “band” theory of bonding in metallic solids.
According to the band theory, any given metal atom has only a limited number of valence electrons with which to bond to all of its nearest neighbors. Extensive sharing of electrons among individual atoms is therefore required. This sharing of electrons is accomplished through overlap of equivalent-energy atomic orbitals on the metal atoms that are immediately adjacent to one another. This overlap is delocalized throughout the entire metal sample to form extensive orbitals that span the entire solid rather than being part of individual atoms. Each of these orbitals lies at different energies because the atomic orbitals from which they were constructed were at different energies to begin with. The orbitals, equal in number to the number of individual atomic orbitals that have been combined, each hold two electrons, and are filled in order from lowest to highest energy until the number of available electrons has been used up. Groups of electrons are then said to reside in bands, which are collections of orbitals. Each band has a range of energy values that the electrons must possess to be part of that band; in some metals, there are energy gaps between bands, meaning that there are certain energies that the electrons cannot possess. The highest energy band in a metal is not filled with electrons because metals characteristically possess too few electrons to fill it. The high thermal electrical conductivities of metals is then explained by the notion that electrons may be promoted by absorption of thermal energy into these unfilled energy levels of the band.
Temperature
|
I |
|
INTRODUCTION |
Temperature, in physics, property of systems that determines whether they are in thermal equilibrium (see Thermodynamics). The concept of temperature stems from the idea of measuring relative hotness and coldness and from the observation that the addition of heat to a body leads to an increase in temperature as long as no melting or boiling occurs. In the case of two bodies at different temperatures, heat will flow from the hotter to the colder until their temperatures are identical and thermal equilibrium is reached (see Heat Transfer). Thus, temperatures and heat, although interrelated, refer to different concepts, temperature being a property of a body and heat being an energy flow to or from a body by virtue of a temperature difference. See Energy.
Temperature changes have to be measured in terms of other property changes of a substance. Thus, the conventional mercury thermometer measures the expansion of a mercury column in a glass capillary, the change in length of the column being related to the temperature change. If heat is added to an ideal gas contained in a constant-volume vessel, the pressure increases, and the temperature change can be determined from the pressure change by Gay-Lussac's law (see Gases), provided the temperature is expressed on the absolute scale.
|
II |
|
TEMPERATURE SCALES |
One of the earliest temperature scales was that devised by the German physicist Gabriel Daniel Fahrenheit. According to this scale, at standard atmospheric pressure, the freezing point (and melting point of ice) is 32° F, and the boiling point is 212° F. The centigrade, or Celsius scale, invented by the Swedish astronomer Anders Celsius, and used throughout most of the world, assigns a value of 0° C to the freezing point and 100° C to the boiling point. In scientific work, the absolute or Kelvin scale, invented by the British mathematician and physicist William Thomson, 1st Baron Kelvin, is most widely used. In this scale, absolute zero is at -273.16° C, which is zero K, and the degree intervals are identical to those measured on the Celsius scale (see Absolute Zero). The corresponding “absolute Fahrenheit” or Rankine scale, devised by the British engineer and physicist William J. M. Rankine, places absolute zero at -459.69° F, which is 0° R, and the freezing point at 491.69° R. A more consistent scientific temperature scale, based on the Kelvin scale, was adopted in 1933.
|
III |
|
EFFECTS OF TEMPERATURE |
Temperature plays an important part in determining the conditions in which living matter can exist. Thus, birds and mammals demand a very narrow range of body temperatures for survival and must be protected against extreme heat or cold. Aquatic species can exist only within a narrow temperature range of the water, which differs for various species. Thus, for example, the increase in temperature of river water by only a few degrees as a result of heat discharged from power plants may kill most of the native fish.
The properties of all materials are also markedly affected by temperature changes. At arctic temperatures, for example, steel becomes very brittle and breaks easily, and liquids either solidify or become very viscous, offering high frictional resistance to flow. At temperatures near absolute zero, many materials exhibit strikingly different characteristics. At high temperatures, solid materials liquefy or become gaseous; chemical compounds may break up into their constituents.
The temperature of the atmosphere is greatly influenced by both the land and the sea areas. In January, for example, the great landmasses of the northern hemisphere are much colder than the oceans at the same latitude, and in July the situation is reversed. At low elevations the air temperature is also determined largely by the surface temperature of the earth. The periodic temperature changes are due mainly to the sun's radiant heating of the land areas of the earth, which in turn convect heat to the overlying air. As a result of this phenomenon, the temperature decreases with altitude, from a standard reference value of 15.5° C (60° F) at sea level (in temperate latitudes), to about -55° C (about -67° F) at about 11,000 m (about 36,000 ft). Above this altitude, the temperature remains nearly constant up to about 33,500 m (about 110,000 ft).
Machine Tools
|
I |
|
INTRODUCTION |
Machine Tools, stationary power-driven machines used to shape or form solid materials, especially metals. The shaping is accomplished by removing material from a workpiece or by pressing it into the desired shape. Machine tools form the basis of modern industry and are used either directly or indirectly in the manufacture of machine and tool parts.
Machine tools may be classified under three main categories: conventional chip-making machine tools, presses, and unconventional machine tools. Conventional chip-making tools shape the workpiece by cutting away the unwanted portion in the form of chips. Presses employ a number of different shaping processes, including shearing, pressing, or drawing (elongating). Unconventional machine tools employ light, electrical, chemical, and sonic energy; superheated gases; and high-energy particle beams to shape the exotic materials and alloys that have been developed to meet the needs of modern technology.
|
II |
|
HISTORY |
Modern machine tools date from about 1775, when the English inventor John Wilkinson constructed a horizontal boring machine for producing internal cylindrical surfaces. About 1794 Henry Maudslay developed the first engine lathe. Later, Joseph Whitworth speeded the wider use of Wilkinson's and Maudslay's machine tools by developing, in 1830, measuring instruments accurate to a millionth of an inch. His work was of great value because precise methods of measurement were necessary for the subsequent mass production of articles having interchangeable parts.
The earliest attempts to manufacture interchangeable parts occurred almost simultaneously in Europe and the United States. These efforts relied on the use of so-called filing jigs, with which parts could be hand-filed to substantially identical dimensions. The first true mass-production system was created by the American inventor Eli Whitney, who in 1798 obtained a contract with the U.S. government to produce 10,000 army muskets, all with interchangeable parts.
During the 19th century, such standard machine tools as lathes, shapers, planers, grinders, and saws and milling, drilling, and boring machines reached a fairly high degree of precision, and their use became widespread in the industrializing nations. During the early part of the 20th century, machine tools were enlarged and made even more accurate. After 1920 they became more specialized in their applications. From about 1930 to 1950 more powerful and rigid machine tools were built to utilize effectively the greatly improved cutting materials that had become available. These specialized machine tools made it possible to manufacture standardized products very economically, using relatively unskilled labor. The machines lacked flexibility, however, and they were not adaptable to a variety of products or to variations in manufacturing standards. As a result, in the past three decades engineers have developed highly versatile and accurate machine tools that have been adapted to computer control, making possible the economical manufacture of products of complex design. Such tools are now widely used.
|
III |
|
CONVENTIONAL MACHINE TOOLS |