
KAPLAN_USMLE_STEP_1_LECTURE_NOTES_2018_BIOCHEMISTRY_and_GENETICS
.pdf

Glycolysis and Pyruvate |
12 |
Dehydrogenase |
Learning Objectives
Answer questions about carbohydrate digestion
Demonstrate understanding of glucose transport
Understand concepts of aerobic and anaerobic glycolysis
Explain information related to galactose metabolism
Explain information related to fructose metabolism
Answer questions about pyruvate dehydrogenase
OVERVIEW
All cells can carry out glycolysis. In a few tissues—most importantly red blood cells—glycolysis represents the only energy-yielding pathway available. Glucose is the major monosaccharide that enters the pathway, but others such as galactose and fructose can also be used.
The first steps in glucose metabolism in any cell are transport across the membrane and phosphorylation by kinase enzymes inside the cell to prevent it from leaving via the transporter.
CARBOHYDRATE DIGESTION
Only a very small amount of the total carbohydrates ingested are monosaccharides. Most of the carbohydrates in foods are in complex forms, such as starch (amylose and amylopectin) and the disaccharides sucrose and lactose.
•In the mouth, secreted salivary amylase randomly hydrolyzes the starch polymers to dextrins (<8–10 glucoses).
•Upon entry of food into the stomach, the acid pH destroys the salivary amylase.
•In the intestine, the dextrins are hydrolyzed to the disaccharides maltose and isomaltose.
•Disaccharides in the intestinal brush border complete the digestion process:
–Maltase cleaves maltose to 2 glucoses
–Isomaltase cleaves isomaltose to 2 glucoses
–Lactase cleaves lactose to glucose and galactose
–Sucrase cleaves sucrose to glucose and fructose
Uptake of glucose into the mucosal cells is performed by the sodium/glucose transporter, an active transport system.
175

Part I ● Biochemistry |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Insulin |
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
ATP *ADP |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
Isomerase |
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PFK–2 |
|
|
Fructose 2, |
||||||
|
|
Glucose |
|
|
Glucose |
|
|
|
|
Glucose-6P |
|
|
|
|
|
Fructose-6P |
|
|
||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||||
|
|
|
|
Mg2+ |
|
|
|
|
|
ATP ADP |
6-bis P |
|||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
Transport |
|
|
Hexokinase |
|
|
|
|
|
|
|
* |
|
ATP |
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||
|
|
|
|
|
|
|
Glucokinase (liver) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ADP |
|
|
PFK–1 + |
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(phosphofructokinase) |
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fructose-1, 6-bis P |
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aldolase |
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Glyceraldehyde-3P |
|
|
|
|
|
|
|
|
|
|
|
|
Dihydroxyacetone-P |
|
||||||||||||||||||
|
|
|
|
NAD |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(DHAP) |
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Isomerase |
|
|
|
|
|
|
|
|
Glycerol-3P |
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
ETC/O2 |
|
|
|
|
Pi |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
Glyceraldehyde 3P |
|
|
|
|
|
|
|
|
|
|
|
dehydrogenase |
|||||||||||||||||||||
|
|
|
Mitochondria |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
dehydrogenase |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
NADH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Glycerol-3P |
|
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
• TGL synthesis |
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
• Electron shuttle |
|
|||||||||
|
|
|
|
|
|
|
|
|
1,3-Bisphosphoglycerate |
|
(RBC) |
|
|
|
||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
ADP |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
Phosphoglycerate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,3-Bisphosphoglycerate |
|
|
|
||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
ATP |
|
kinase |
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3-Phosphoglycerate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mutase |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2-Phosphoglycerate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Enolase |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pyruvate kinase deficiency |
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
Phosphoenolpyruvate (PEP) |
|
|
|
|
|
|
• Hemolytic anemia |
|
|
|
|
|
|
|||||||||||||||||||||
|
|
|
|
|
|
|
|
|
ADP |
|
* |
|
|
|
|
|
|
|
• Increased BPG |
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
Pyruvate kinase |
|
• No heinz bodies |
|
|
|
|
|
|
||||||||||||||||||||||||
|
|
NAD |
NADH |
|
|
ATP |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
–O2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mitochondria |
|||||||||||||||
|
|
|
|
|
|
|
Cytoplasm |
|
|
|
|
|
|
|
+O2 |
|
|
|
|
|
Pyruvate |
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pyruvate dehydrogenase |
|||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
Pyruvate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acetyl-CoA |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Lactate |
|
|
|
Lactate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
or |
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
dehydrogenase |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TCA |
|
|
|
Fatty acid |
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
synthesis |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
*Controlled enzymes catalyzing irreversible steps |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ATP |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
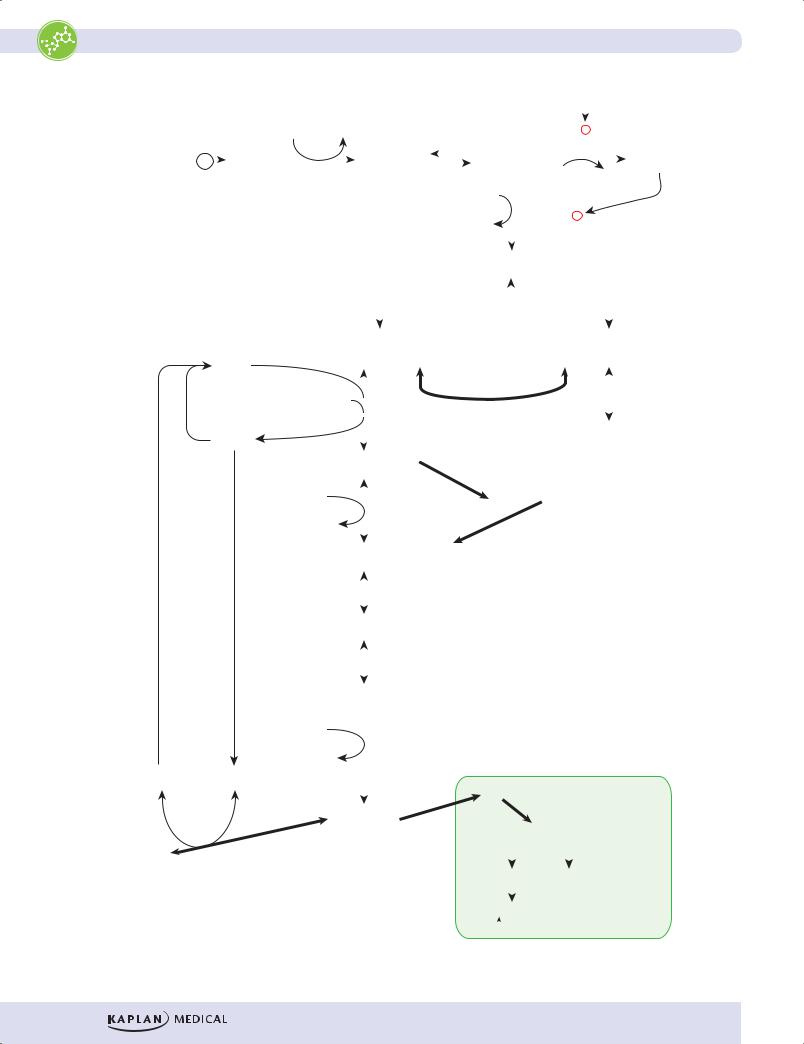
Figure I-12-3. Glycolysis |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
178


Part I ● Biochemistry
•3-phosphoglycerate kinase
–3-phosphoglycerate kinase transfers the high-energy phosphate from 1,3-bisphosphoglycerate to ADP, forming ATP and 3-phosphoglycerate.
–This type of reaction, in which ADP is directly phosphorylated to ATP using a high-energy intermediate, is referred to as a substratelevel phosphorylation.
–Unlike oxidative phosphorylation in mitochondria, substrate-level phosphorylations are not dependent on oxygen, and are the only means of ATP generation in an anaerobic tissue.
•Pyruvate kinase
–The last enzyme in aerobic glycolysis, pyruvate kinase catalyzes a substrate-level phosphorylation of ADP using the high-energy substrate phosphoenolpyruvate (PEP).
–Pyruvate kinase is activated by fructose 1,6-bisphosphate from the PFK-1 reaction (feed-forward activation).
•Lactate dehydrogenase
–Lactate dehydrogenase is used only in anaerobic glycolysis. It reoxidizes NADH to NAD, replenishing the oxidized coenzyme for glyceraldehyde 3-phosphate dehydrogenase.
–Without mitochondria and oxygen, glycolysis would stop when all the available NAD had been reduced to NADH. By reducing pyruvate to lactate and oxidizing NADH to NAD, lactate dehydrogenase prevents this potential problem from developing.
–In aerobic tissues, lactate does not normally form in significant amounts. However, when oxygenation is poor (skeletal muscle during strenuous exercise, myocardial infarction), most cellular ATP is generated by anaerobic glycolysis, and lactate production increases.
180


Part I ● Biochemistry
Recall Question
Which of the following transporters increases its uptake of glucose in response to insulin?
A.Glut 1
B.Glut 2
C.Glut 3
D.Glut 4
Answer: D
ATP Production and Electron Shuttles
Anaerobic glycolysis yields 2 ATP/glucose by substrate-level phosphorylation. Aerobic glycolysis yields these 2 ATP/glucose plus 2 NADH/glucose that can be utilized for ATP production in the mitochondria; however, the inner membrane is impermeable to NADH.
Cytoplasmic NADH is reoxidized to NAD and delivers its electrons to one of 2 electron shuttles in the inner membrane. In the malate shuttle, electrons are passed to mitochondrial NADH and then to the electron transport chain. In the glycerol phosphate shuttle, electrons are passed to mitochondrial FADH2.
•Cytoplasmic NADH oxidized using the malate shuttle produces a mitochondrial NADH and yields approximately 3 ATP by oxidative phosphorylation.
•Cytoplasmic NADH oxidized by the glycerol phosphate shuttle
produces a mitochondrial FADH2 and yields approximately 2 ATP by oxidative phosphorylation.
Glycolysis in the Erythrocyte
In red blood cells, anaerobic glycolysis represents the only pathway for ATP production, yielding a net 2 ATP/glucose.
182


 vesicles with
vesicles with 
 membrane-bound
membrane-bound 
 GLUT 4
GLUT 4  transporters
transporters  Fusion of vesicles
Fusion of vesicles


 membrane Exocytosis
membrane Exocytosis 