
Part III - Well stimulation methods
.pdf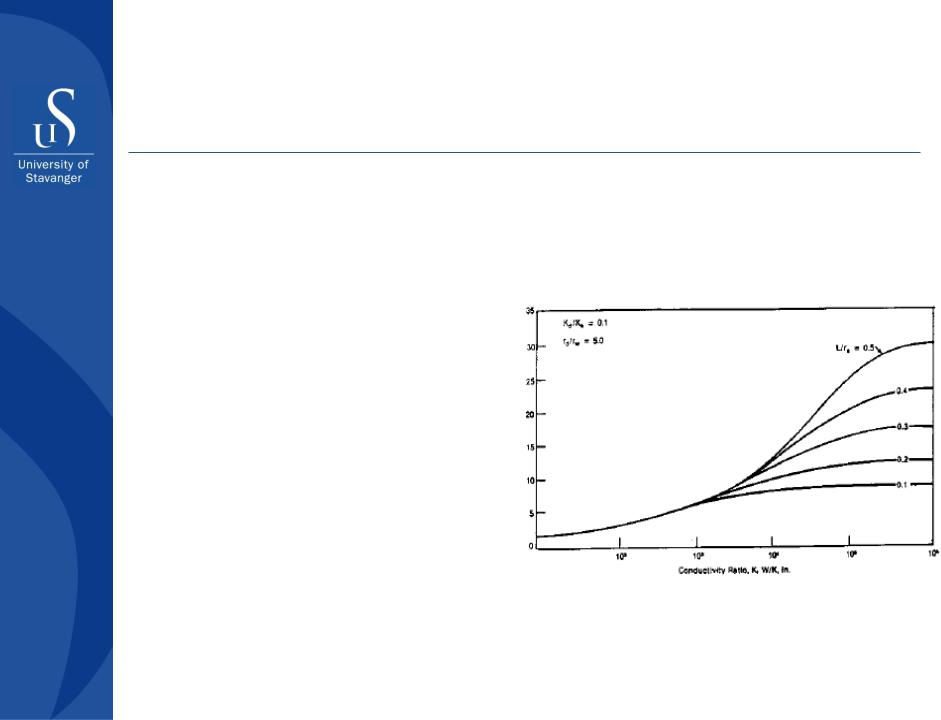
Hydraulic fracturing
Evaluating effect of hydraulic fracturing (McGuire and Sikora, 1960)
Stimulation Ratio, J/J0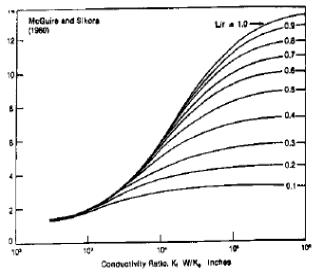
Part III - Well stimulation methods |
21 |
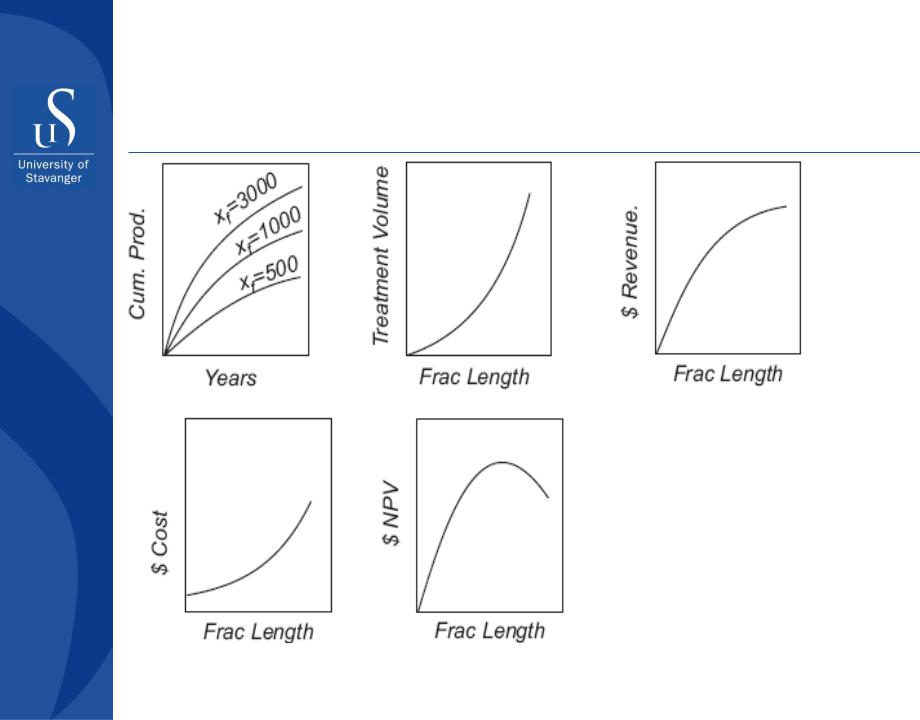
Hydraulic fracturing
Optimization of NPV
Part III - Well stimulation methods |
22 |

Hydraulic fracturing
Different approaches to the hydraulic fracture modeling
I. Perkins, Kern (1961), Nordgren (1972) – PKN-model
Assumption: |
|
|
|
|
|
|
|
|
|
E 3q x |
f |
1/ 4 |
|
||
|
h f p |
|
p |
|
|||
|
|
|
|
|
|||
w |
|
|
|
|
|
||
h f |
|
|
|
||||
E |
|
|
|
|
|
||
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
II. Khristianovich, Zheltov (1955), Geertsma, DeClerk (1969) – KGD-model
Assumption: |
|
|
|
|
|
|
|
|
E 3q 1/ 4 |
|
|||
w |
x f p |
|
p |
|
||
|
|
|
1/ 4 x f 1/ 2 |
|
||
E |
h f |
|
||||
|
|
|
|
|||
|
|
|
|
|
|
|
Part III - Well stimulation methods |
23 |
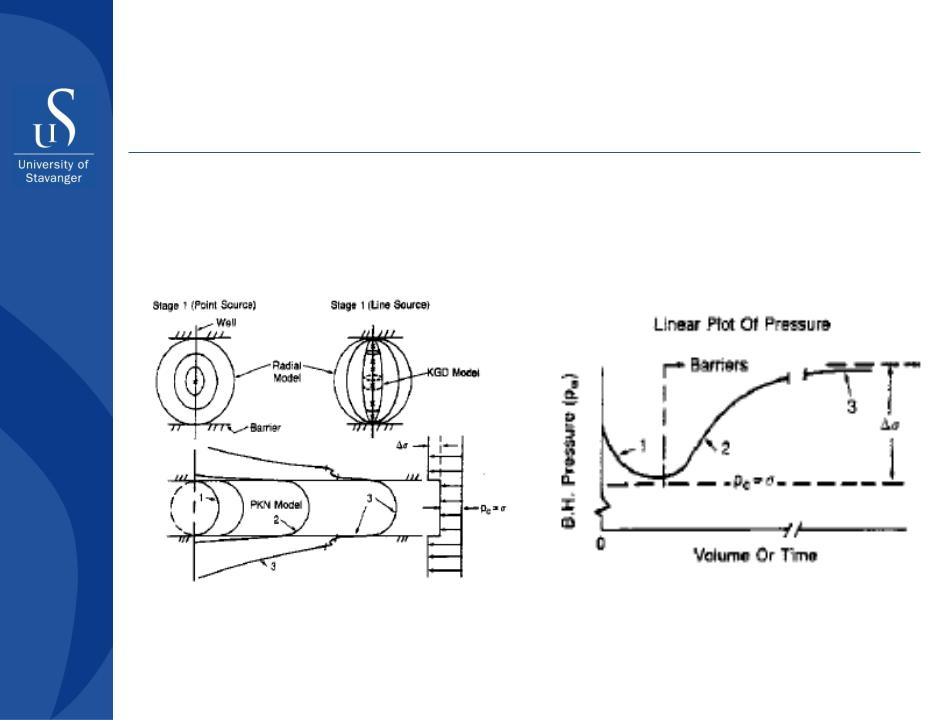
Hydraulic fracturing
Fracture propagation and different approaches to the hydraulic fracture modeling
Part III - Well stimulation methods |
24 |

Matrix Acidizing and
Acid Fracturing
Part III - Well stimulation methods |
25 |

Matrix Acidizing and Acid Fracturing
Reaction kinetics
The major components controlling the kinetics of acid flow and attack in a reactive environment are:
Convective flux of the stimulation fluid into the formation
Diffusive flux caused by concentration gradient from the bulk of the acid solution to the reactive surface
Reaction at the reactive surface
Part III - Well stimulation methods |
26 |

Matrix Acidizing and Acid Fracturing
Reaction kinetics
Type of reaction kinetics is determined by the reaction rate constant k
|
|
E |
|
k k0 exp |
|
|
|
|
|||
|
|
RT |
|
|
|
|
|
Part III - Well stimulation methods |
27 |
Matrix Acidizing and Acid Fracturing
Reaction kinetics
Usually two types of reaction kinetics are distinguished:
Surface reaction limited kinetics
–Example: sandstone dissolution with HF / HCl mixtures, reaction of dolomite with HCl at low temperatures (T<50 0C)
Diffusion limited or mass-transfer limited kinetics
–Example: limestone dissolution with HCl at temperatures >0 0C, reaction of dolomite with HCl at high temperatures (T> 50 0C)
Part III - Well stimulation methods |
28 |
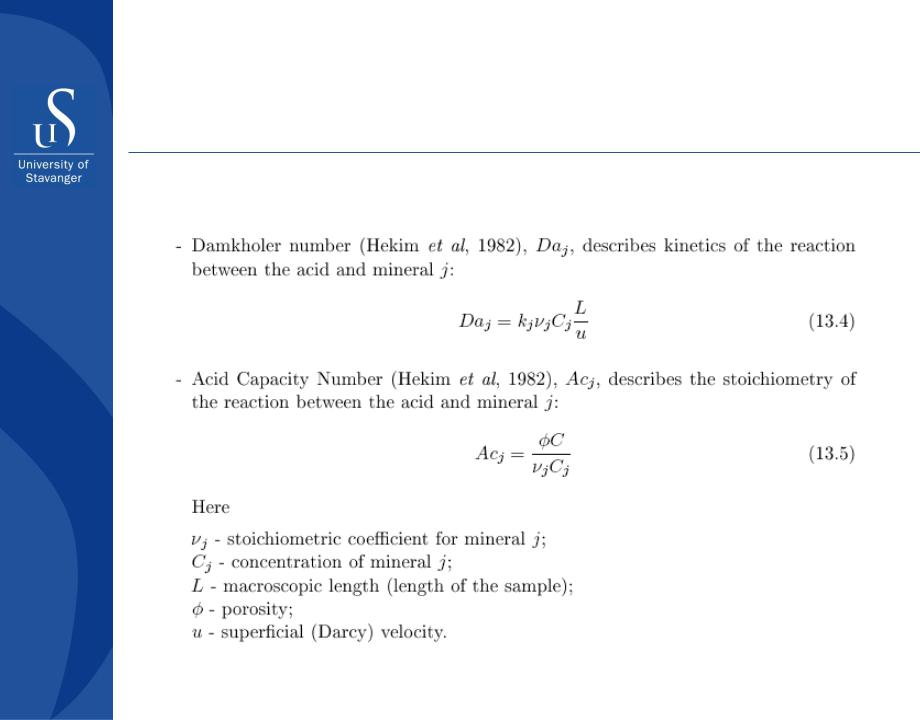
Surface Reaction Limited Kinetics (SRLK)
There are two dimensionless parameters governing SRLK:
Part III - Well stimulation methods |
29 |
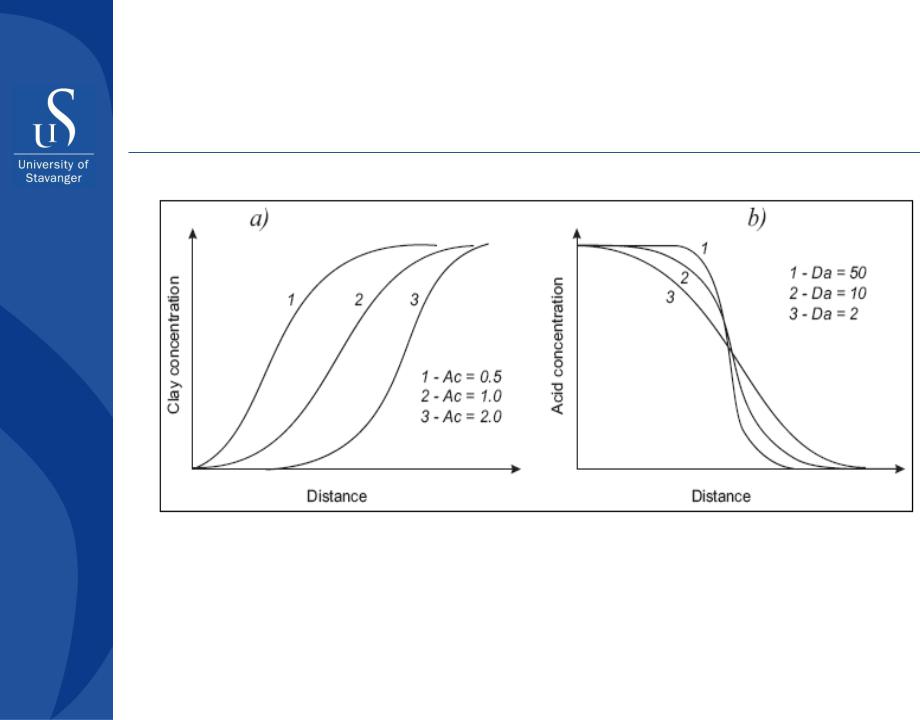
Surface Reaction Limited Kinetics (SRLK)
Dependency of the front location and its sharpness on the acid capacity number Ac and Damkholer number Da
Part III - Well stimulation methods |
30 |
