
Frantisek Svec - Capillary Electrochromatography
.pdf
214 |
S. Imamoglu |
–Eluent consumption, since this determines the cost for mobile phase in terms of preparation and handling (tanks, water preparing systems, pumps)
–Product dilution, since this determines the cost for further product processing (e.g., concentration, polishing) [10].
To optimize a given preparative chromatographic process (highest productivity, lowest mobile phase consumption,and product dilution) the separation has to be performed with the highest product concentrations still compatible with the system. One immediate consequence of this is that each column has to be operated in the non-linear range of the adsorption isotherm.
A counter current movement of the mobile phase and the sorbent has some unique advantages when designing separation processes for maximum economy. The efficiency requirement for the sorbent is lower compared to other chromatographic modes, since no individual column has to achieve full resolution. Instead only the pure fractions of the zones obtained are withdrawn from the system. The time-space yield in terms of productivity is enhanced considerably by the improved utilization of the sorbent capacity. The product dilution is lower, pure fractions are withdrawn with high yield and it is not necessary to consider fractions of less then the desired purity. Early on it was re-
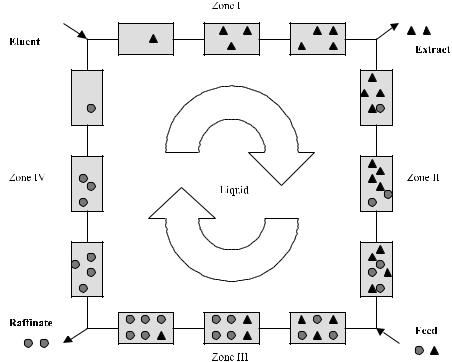
Fig. 1. Schematic description of the different zones in an SMB system and the adsorption and desorption processes in these zones
Simulated Moving Bed Chromatography (SMB) for Application in Bioseparation |
215 |
cognized however, that it is extremely difficult to operate a true moving bed (TMB) system because it would involve the circulation of a solid adsorbent, which would lead to significant mechanical stress for the solid phase. It was soon shown, that all the theoretical advantages of TMB chromatography could also be achieved by the SMB-approach, which uses several fixed-bed columns in series and an appropriate shift of the injection and collection points to simulate the movement of the solid phase. SMB is a continuous process and hence can be perfectly implemented into all continuous production processes. SMB is also much more suited to large-scale production than conventional (batch) chromatography.Another distinct advantage of the SMB-approach is that it generally requires significantly less eluent than other chromatographic separation modes
The classical moving bed consists of four different zones, in which different constraints must be met, Fig. 1:
–Zone I (between the eluent and the extract): the more firmly retained product (A, extract) must be completely desorbed.
–Zone II (between the extract and the feed): the less firmly retained product (B, raffinate) must be completely desorbed.
Fig. 2. Principle of the SMB

216 |
S. Imamoglu |
–Zone III (between the feed and the raffinate): the more firmly retained product (A, extract) must be completely adsorbed.
–Zone IV (between the raffinate and the eluent): the less firmly retained product (B, raffinate) must be completely adsorbed.
Under these circumstances, all the internal flow rates (volumetric flow rates, Q) are related to the inlet/outlet flow rates by simple mass balances:
QII =QI –QExt |
(1) |
QIII =QII +QFeed |
(2) |
QIV =QIII –QRaff |
(3) |
QI =QIV +QEl |
(4) |
Fig. 3. General set-up of a SMB separation system
Simulated Moving Bed Chromatography (SMB) for Application in Bioseparation |
217 |
The inlet/outlet flow rates are related by:
QExt +QRaff =QFeed +QEl |
(5) |
Between Zone II and III, the feed mixture is introduced into the system and transported with the mobile phase into Zone III, Fig. 2.
In Zone III the compounds which have higher affinity to the sorbent are adsorbed and transported with the stationary phase to Zone I. There they are desorbed by a mixture of fresh eluent introduced between Zones I and IV and the recycled eluent from Zone IV. The less adsorbed compounds in Zone III are moved with the mobile phase to Zone IV. There they adsorb and are transported in that form together with the stationary phase (column) to Zone II, where they finally become desorbed.
The different adsorption and desorption events are controlled via the flow rates adjusted by the means of 3 or 5 external pumps and the column switch times, Fig. 3. The key element for success is the proper selection of the respective flow rates, which must be chosen in such a way that the extract front between zones I and II and the raffinate front between zones III and IV are stabilized, while the separation between zones II and III is assured. A simple trial-and- error approach to such an optimization of the system parameters is unlikely to be successful. Instead, the chromatographic behavior of all compounds has to be modeled and simulated.
In the first step, the adsorption isotherms of the compounds should be determined under non-linear chromatographic conditions, which can be done in several ways [11].Afterwards, models should be implemented and used to simulate the chromatographic behavior and to find the optimum system parameters for a given separation problem. Different approaches for finding the optimum parameter are described in the literature [12–16] mainly for adsorption and ion exchange chromatography.
2.1
Technical Aspects of SMB Implementation
A classical Simulated Moving Bed system consists of 4 to 24 columns distributed between 4 zones, in addition to 3 to 5 pumps and valves which connect the different streams between the columns. In general a 4 column SMB should be sufficient to test and optimize the conditions for any given separation problem. The optimal number of columns per zone must be determined in the simulation of the SMB process. The rule is more columns per zone result in a better separation, while too many columns per zone make the system too complex. If an infinite number of columns per zone are used the SMB approaches a TMB.
There are different ways to connect the columns to build a SMB system.An important aspect is always the position of the recycling pump. The recycling pump ensures the internal flow of the mobile phase. Most often the recycling pump is placed between the last and the first column,i.e. columns 12 and 1 in Fig. 2. Once the recycling pump is fixed with respect to the columns, it moves with respect to the zones and is alternatively located in zones IV, III, II, and I. The flow rates required in the different zones are different and so the pump flow rates vary from
218 |
S. Imamoglu |
zone to zone. With small variations, most large-scale SMB-units show this basic design. Process control is comparatively easy under these circumstances, since the design of the system is relatively simple. For small SMB-systems the volume of the recycling pump can lead to an asymmetry,which in turn can result in a decrease in final purity. Possible solutions to that problem are the use of a shorter column (near the recycling pump) or an asynchronous shift of the inlets and outlets [17].
Another option for the recycling pump is to fix it with respect to the zones rather than the columns. In this case the pump is always located between zone IV and I where only the eluent is present. However, since the columns rotate, additional valves are needed for this design, which makes the system more complex. A third possibility is to use an eluent pump instead of a recycling pump. Again this variant requires more valves than, e.g., the first option and has the disadvantage of requiring the connection of one outlet to the eluent reservoir. Independent of the location of the recycling pump,there are different options to control the outlet flow rates. In particular this can be done by pumps, by analogous valves, by flow meters, or by pressure control.
Summing up,a robust and easy to handle SMB-design uses 4 zones,a recycling pump fixed in respect to the columns and two pumps for the control of the outlet flow rates. Extremely high precision of all technical components of the SMB is needed. All pumps and valves have to be exactly synchronized. The flow rates should not vary by more than1% from the preset value.All connections between the different parts of the system must be carefully optimized in order to minimize the dead volume. All columns should be stable and nearly identical in performance. If the SMB-technology is to be used in Biotechnology,GMP issues (cleaning, process and software validation) also have to be considered. In addition and as with any continuous process in that particular area, the definition of a batch could be a problem.
2.2
Operating Conditions
The important step in designing an SMB is to find the operating conditions suited to processing a given amount of feed per day or week or month [18]. The procedure, which is illustrated here, is based on the modeling of non-linear chromatography. Since the SMB is to be used as a preparative separation technique, which is supposed to operate under high product overload conditions, i.e. in the non-linear part of the adsorption isotherm, the linear part of the adsorption isotherm is normally of little importance. SMBs can also be operated under linear conditions but this somewhat academic case will only be considered marginally here. The determination of the competitive non-linear adsorption isotherms of both compounds of interest is, on the other hand, generally required. It is usually possible to get all relevant data defining suitable operating conditions from measuring the feed mixture directly. The use of the pure components is usually not necessary [18].
As mentioned previously, the design of an SMB-separation requires the correct choice of the different flow rates of the recycle stream, the feed stream, the
Simulated Moving Bed Chromatography (SMB) for Application in Bioseparation |
219 |
eluent stream,the extract stream,the raffinate stream and the shift period for the columns/zones, which corresponds to the simulated “solid stream”. Other important parameters for the operating conditions are:
–The feed concentrations
–The number of columns per zone
–The column length
–The column diameter and
–The particle size.
All these parameters can be determined and optimized by data measuring on the laboratory scale.
3
Theoretical Background
3.1
The “Triangle Theory”
For a deeper understanding of SMB behavior, a more synthetic view of the process is required. This is, e.g., possible by applying the Equilibrium Theory Model, i.e. a model where mass transfer resistance and axial dispersion are neglected (columns of infinite efficiency). The application of this highly idealized model to SMB units under the concomitant assumption of Langmuir-type adsorption isotherms forms the basis of the so-called “Triangle Theory” proposed by Morbidelli and his group [19]. The Triangle theory facilitates the determination of optimal and robust operating conditions of SMBs suitable for achieving the desired separation [20–27]. A major feature of this approach consists of the fact that the typical overloaded operating conditions of the SMB can be taken into account, i.e. the highly non-linear and competitive adsorption behavior. This makes this approach superior to particularly when compared with others,which are based on empirical extrapolations of the linear adsorption isotherms to design the non-linear SMB operations [28].
Let us assume a standard four-zone SMB unit, in which the complete separation of a binary mixture, constituted of the more retained component A and the less retained component B is to be achieved. In the framework of the Equilibrium Theory,the key operating parameters through which the performance of the SMB can be controlled are the flow rate ratios, mj , j=1,…,4, in the four sections of the SMB unit, according to:
mj = Qj t*–Ve* |
(6) |
395V(1–e*) |
|
where V is the column volume, t* is the column switch time,i.e.,the time between two successive switches of the inlet and outlet ports, e*=e+(1–e) ep is the overall void fraction of the column,with e and ep ,being the bed void fraction and the macroporosity of the stationary phase particles,and Qj is the volumetric flow rate in the jth section of the SMB unit.

220 S. Imamoglu
Constraints on the values of the flow rate ratios can thus be determined,which depend solely on parameters characterizing the adsorption equilibrium of the species to be separated. In the most general case, these can be derived from a biLangmuir multicomponent adsorption isotherm [27] or,as previously suggested, from the Langmuir and the modified Langmuir isotherm [20,25]. For the sake of simplicity the binary Langmuir isotherm will be used from hereon,as defined by:
ni = |
Hi ci |
, i=A, B |
(7) |
||
c |
+K c |
||||
1+K |
|
|
|||
36892 |
|
|
|||
|
A A |
B B |
|
|
|
where ni and ci are the adsorbed and mobile phase concentration,Hi is the Henry constant of the ith component, i.e. the slope of the single component adsorption isotherm at infinite dilution,Ki is the equilibrium constant of the ith component, which accounts for the competitive and overload effects.
Subsequently,the condition of complete separation has to be coupled with the material balances derived for the nodes of the SMB unit and implemented in the Equilibrium Theory Model for Langmuir-type systems. That leads to the set of mathematical conditions given below, which the flow rate ratios have to fulfil in
order to achieve complete separation, in particular: |
|
|
||||||
HA <m1 <• |
|
|
|
|
(8) |
|||
m2, cr (m2 , m3)<m2 <m3 <m3, cr (m2 , m3) |
|
(9) |
||||||
–ep |
|
<m4 <m4, cr (m2 , m3) |
|
(10) |
||||
1–ep |
|
|||||||
63 |
|
|
|
|
|
|
|
|
= 1 |
{ |
H |
+m3 |
+K |
cF (m3 |
–m2)– [HB+m3+KB cBF (m3 |
–m2]2 |
–4 HB m3 |
2 |
B |
|
B |
B |
9999959} |
|||
|
|
|
|
|
|
|
|
|
where the superscript F indicates the feed conditions.
The constraints on m1 and m4 are explicit. The lower limit of m1 , however, does not depend on the other flow rate ratios,whereas the upper limit of m4 is an explicit function of the flow rate ratios m2 and m3 and of the feed composition respectively [25]. The constraints on m2 and m3 are implicit (see Eq. 4), but they do not depend on m1 and m4. Therefore, they define a unique region of complete separation in the (m2 , m3) plane, which is the triangle-shaped region abw in Fig. 4. The boundaries of this region can be calculated explicitly in terms of the adsorption equilibrium parameters and the feed composition as follows [25]:
– Straight line wf:
(HA –wG (1+KA cAF)) m2 +KA cAF wG m3 =wG (HA –wG) . |
(11) |
|||||||||||||
– Straight line wb: |
|
|
|
|
|
|
|
|
|
|
|
|||
(H |
A |
–H |
(1+K |
cF)) m |
+K |
cF H |
B |
m |
=H |
(H |
A |
–H |
) . |
(12) |
|
B |
|
A A 2 |
|
A A |
3 |
B |
|
B |
|
|
|||
– Curve ra: |
|
( 5HA – 5m2)2 |
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
||
m3 =m2+ |
F . |
|
|
|
|
|
|
|
|
(13) |
||||
|
|
|
36832Ka cA |
|
|
|
|
|
|
|
|
|
|
|

Simulated Moving Bed Chromatography (SMB) for Application in Bioseparation |
|
221 |
||
– Straight line ab: |
|
|
|
|
m3 =m2 . |
|
|
|
(14) |
The co-ordinates of the intersection points are given by: |
|
|
||
point a(HA , HA) |
|
|
(15) |
|
point b(HB , HB) |
|
|
(16) |
|
point f(wG , wG) |
|
|
(17) |
|
point r wG2 |
, wG [wF (HA –wG) (HA –HB)+HB wG (HA –wF)] |
(18) |
||
HA |
|
HA HB (HA –wF) |
|
|
6 |
999199999 |
|
||
and point w HB wG , wG [wF (HA –HB)+HB (HB –wF)] |
|
(19) |
||
|
HA |
HB (HA –wF) |
|
|
|
65 |
99919939 |
|
|
In the above equation wF and wG depend on the feed composition. They are the roots of the following quadratic equation, with wG >wF >0:
(1+KA cAF +KB cBF) w2 –[HA (1+KB cBF)+HB (1+KA cAF)] w+HA HB =0 . (20)
As illustrated in Fig. 4, the region of complete separation is surrounded by three regions corresponding to three different operating regimes. In the region
Fig. 4. Separation of a two component mixture using a non-adsorbable desorbent/eluent
222 |
S. Imamoglu |
of pure raffinate, as the name indicates, the raffinate stream is pure but the extract is polluted by component B. In the region of pure extract,the extract is pure, but the raffinate is polluted by component A. In the third region (no pure fraction), both components A and B are found in both the extract and the raffinate streams. The information from the geometrical representation of the separation regions in the (m2 , m3) plane in Fig. 4 is only correct if the relevant constraints on m1 and m4 are fulfilled, in particular inequalities (8) and (10).
The vertex w of the region of complete separation in the (m2 , m3) plane represents the optimal operating conditions in terms of solvent consumption and productivity per unit mass of stationary phase. However, under such circumstances, even the slightest disturbance in the process conditions or the smallest error in the evaluation of the adsorption equilibrium parameters will result in a slight deviation of the operating point from the optimal location into a region, where complete separation is no longer possible. Since the optimal operating conditions are not very robust,the operating point under realistic conditions is chosen somewhere inside the complete separation triangle and not at its vertex. That is a compromise between separation performance (productivity and solvent requirement) and robustness of the performance.
The multicomponent Langmuir adsorption isotherm given in Eq. (7) is the simplest model for the description of non-linear, multicomponent, adsorption equilibrium. At high concentration, the model predicts “saturation” of the stationary phase and overload of the chromatographic column. At low concentration (high dilution) the behavior can be correctly described by the non-compet- itive linear adsorption isotherm:
ni =Hi ci (i=A, B) . |
(21) |
When translated to the SMB conditions, these features imply that increasing feed concentration lead to an increasing degree of non-linearity due to the fact that the adsorption columns increasingly are operated under overload conditions. This effect is predicted by the approach summarized in the previous section, in particular by Eqs. (8) to (19), which allow the calculation of the constraints on m1 and m4 and the boundaries of the complete separation region in the (m2 , m3) plane as a function of feed composition [19].
The non-linearity effect can easily be demonstrated by the following theoretical separation of a binary mixture. Let us assume that the concentrations of A and B are the same and correspond each to half of the overall feed concentration. The feed concentration is in addition assumed to be the only parameter necessary to characterize the feed composition. The mass flow ratio in section 1 (constrained by Eq. (8)) does not depend on the feed composition. On the contrary, the upper limit on the flow rate ratio m4 given by Eq. (10) is a function of the feed composition. Both dependencies are illustrated in Fig. 5.
When the constraint on m4 is not fulfilled, some of the weakly adsorbed component B is carried over by the recycled mobile phase and starts to pollute the extract.
Figure 6, on the other hand, illustrates the differences between operating an SMB under linear and non-linear conditions. In particular, this figure illustrates the effect of the overall concentration on the region of complete separation re-

Simulated Moving Bed Chromatography (SMB) for Application in Bioseparation |
223 |
|
|
|
|
|
|
|
Fig. 5. Optimal values of the flow rate ratios as a function of the overall feed concentration
Fig. 6. Effect of the overall concentration of the feed mixture on the region of complete separation
