
Modern Organocopper Chemistry
.pdf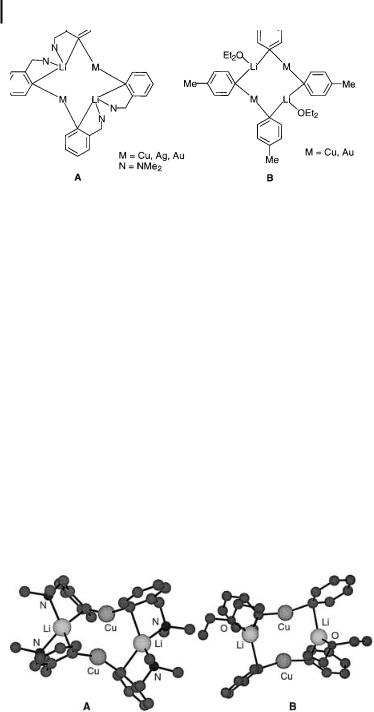
28 1 Structures and Reactivities of Organocopper Compounds
Fig. 1.23. Proposed structures in solution for M2Li2(C6H4CH2NMe2-2)4 (A) and M2Li2(C6H4Me-4)4(OEt2)2 (B).
showed that each of the Cipso atoms is coupled to one Li atom (1Jð13C; 7LiÞ ¼ 7:2 Hz) and to one Ag atom (1Jð13C; 109AgÞ ¼ 136 Hz). The 6Li, 7Li, and 109Ag NMR
spectra pointed to the presence of one type of silver and one type of lithium atom, with each lithium atom being coupled to two silver atoms and each silver atom coupled to two lithium atoms (2Jð7Li; 109AgÞ ¼ 3:91 Hz) [107].
On the basis of these data and of spectroscopic similarities between the Cu, Ag, and Au compounds, a structure for these compounds was proposed. This is shown schematically in Fig. 1.23A. This dimeric aggregated structure is not a consequence of the ortho functionalization of the aryl anion with a coordinating heteroatom substituent, as became evident from studies of the structural features of the simple arylcuprate Cu2Li2 (C6H4Me-4)4(OEt2)2 and the corresponding gold compound in solution [108]. For these aggregated species, a structure similar to that of M2Li2(C6H4CH2NMe2-2)4 was proposed (see Fig. 1.23B). However, the lithium
atoms are now trigonally coordinated. |
|
|
|
The solid state structures of Cu2Li2(C6H4CH2NMe2-2)4 |
(Fig. |
1.24A) |
[72], |
Au2Li2(C6H4CH2NMe2-2)4 [109], and Cu2Li2(C6H5)4(OEt2)2 |
(Fig. |
1.24B) |
[110], |
Fig. 1.24. Structures of Cu2Li2(C6H4CH2NMe2-2)4 (A) and Cu2Li2(C6H5)4(OEt2)2 (B) in the solid state.

1.4 Organocuprates 29
were later established by X-ray crystal structure determination and appeared to be in full agreement with the corresponding structures in solution as deduced from spectroscopic data.
Cu2Li2(C6H4CH2NMe2-2)4 was the first neutral cuprate for which the structure could be unambiguously established. A common feature of such structures is the bridging nature of the aryl group. As a consequence of the binding of Cipso to two di erent metal atoms, this bridging (in comparison with that in homoleptic organocopper compounds) is unsymmetrical (shorter CuaC bond, longer LiaC bond). This asymmetry is even more pronounced in the corresponding gold compound [109], which is best described as consisting of two R2Au anionic units linked together by solvated lithium cations through contact ion-pair formation. Another consequence of the bridging between two di erent metal atoms is the fact that, if the aryl group is not symmetrically substituted, the bridging carbon atom becomes a center of chirality. This has important stereochemical consequences [111]. A particularly complicated situation arises if the benzylic group in, for example, Cu2Li2(C6H4CH2NMe2-2)4 bears a substituent that gives rise to the presence of di erent diastereoisomeric units in one aggregate [111]. The pure organocopper compound Cu4(C6H4CH(Me)NMe2-2)4, and the corresponding cuprate, Cu2Li2(C6H4CH(Me)NMe2-2)4, were prepared starting both from the enantiopure ligand and from the racemic ligand. It appeared that the organocopper compound Cu4(C6H4CH(Me)NMe2-2)4 derived from the racemic ligand exists in solution as a mixture of all possible diastereoisomeric aggregates. This observation, however, contrasts with the situation found for the cuprate Cu2Li2(C6H4CH(Me)NMe2-2)4 derived from the racemic ligand. In this case, only aggregates in which all four bridging and benzylic carbon centers have the same relative stereochemical configuration are formed, in a nice example of diastereoselective self-assembly [112].
That the natures both of the organic group and of the additional donor-solvent molecules are factors that determine the actual cuprate aggregates formed is demonstrated by the structure of [Cu2Li2(CH2SiMe3)4(DMS)2]n in the solid state [113] (see Fig. 1.25). In this structure, the basic framework consists of repeating central
Fig. 1.25. Structure of [Cu2Li2(CH2SiMe3)4(DMS)2]n in the solid state.

30 1 Structures and Reactivities of Organocopper Compounds
Fig. 1.26. Structure of Cu2Li3Ph5(DMS)4 in the solid state.
Cu2Li2 cores with bridging Me3SiCH2 groups. These Cu2Li2 cores are interlinked to form linear chains through bridging Me2S ligands between the lithium atoms of two adjacent Cu2Li2 cores.
The solid-state structure of Cu2Li2Ph4(DMS)3 is closely related to that observed for Cu2Li2Ph4(OEt2)2, except that one of the lithium atoms here is now fourcoordinate as a result of coordination of two DMS molecules [114]. This observation shows that even slight changes in the coordinating properties of donor solvent molecules may change the overall structure of the cuprate.
So far, only cuprates with a 1:1 copper/lithium ratio have been considered. Treatment of phenyllithium with various substoichiometric quantities of copper bromide in DMS as solvent a orded so-called higher order cuprates, of which two were characterizable by X-ray crystallography. These have the overall stoichiometries Cu2Li3Ph5(DMS)4 and Cu4Li5Ph9(DMS)4 [114, 115]. The structure of the former compound in the solid state is shown in Fig. 1.26.
The Cu4Li5Ph9(DMS)4 aggregate may be described as consisting of three linear CuPh2 anions, triply bridged by two lithium cations, and of one trigonal Ph3Cu2 anion, which is associated with three lithium cations and coordinated by four DMS ligands. The two resulting units – [(CuPh2)3Li2] and [(CuPh3)Li3(DMS)4]þ – are linked together by a bridging phenyl group ipso carbon atom.
The only examples of cuprates in which copper and magnesium atoms are incorporated in one aggregate have the stoichiometries Cu2MgPh4(THF)n [60], Cu5Mg2Ph4Br5(THF)n [60], Cu4Mg(PhMe-4)6(OEt2) [116], and Cu4MgPh6(OEt2) [116]. The structure of Cu4MgPh6(OEt2) in the solid state (Fig. 1.27) was established by X-ray crystal structure determination [117].
The structure of Cu4MgPh6(OEt2) comprises a central core of five metal atoms in a trigonal bipyramidal arrangement, with the magnesium atom at an axial position. The six phenyl groups bridge across the axial–equatorial edges of the trigonal bipyramid. One diethyl ether molecule is coordinated to the magnesium atom,

1.4 Organocuprates 31
Fig. 1.27. Structure of Cu4MgPh6(OEt2) in the solid state.
to attain coordination saturation. A similar arrangement of the metal framework is observed in the anions [Cu5Ph6] and [Cu3Li2Ph6] , discussed in the next section.
For reasons outlined above, the neutral heteroleptic cuprates ‘‘RCuLiX’’ (X ¼ heteroatom-containing ligand) are valuable reagents in organic synthesis. Despite this importance, however, only a very few have been structurally characterized. The structure of CuLiMe(t-Bu2P)(THF)3 has been established by X-ray crystal structure determination [118] (Fig. 1.28A). The copper atom has a linear CaCuaP geometry. In contrast to most other cuprates, in which lithium is involved in twoelectron, three-center bonding with the organic group, the lithium atom is bound in this case to the heteroatom anion (P) and three THF molecules.
Another example of a neutral, heteroleptic cuprate is the arylcopper magnesium arenethiolate [Cu4Mes4][Mg(SC6H4CH(Me)NMe2-2)2]2 (Fig. 1.28B), formed by self-assembly in solutions of Cu3(SC6H4CH(Me)NMe2-2)3 and Mes2Mg [93]. This copper complex may be regarded as a model compound for a possible active species
Fig. 1.28. Structures of heteroleptic cuprates CuLiMe(t-Bu2P)-(THF)3 (A) and [Cu4Mes4] [Mg(SC6H4CH(Me)NMe2-2)2]2 (B) in the solid state.

32 1 Structures and Reactivities of Organocopper Compounds
when Cu3(SC6H4CH(Me)NMe2-2)3 is used as a catalyst in enantioselective 1,4- addition reactions of Grignard reagents to enones [91, 92].
The formation of heteroleptic organocuprates ‘‘CuLiRR’’’ in solution has been proposed in, for example, the reaction in diethyl ether between enantiopure Cu4(C6H4CH(Me)NMe2-2)4 and Li4Me4 (Eqn. 1 in Scheme 1.22) to a ord a heteroleptic cuprate Cu2Li2(C6H4CH(Me)NMe2-2)2(Me)2. These solutions were applied in 1,4-addition reactions. Although methyl transfer to the substrate occurs, no enantioselective induction is observed [119]. A possible explanation for this lack of stereoselectivity involves the occurrence of a disproportionation reaction of the heteroleptic cuprate into the corresponding homoleptic cuprates Cu2Li2(C6H4CH- (Me)NMe2-2)4 and Cu2Li2Me4. In the authors’ laboratory, the reaction between the corresponding achiral organocopper compound Cu4(C6H4CH2NMe2-2)4 and Li4Me4 in various ratios has been studied. 1H NMR spectroscopy has shown that such solutions are complicated equilibrium mixtures (Eqn. 2 in Scheme 1.22) of several aggregates, of which the homoleptic cuprates Cu2Li2(C6H4CH2NMe2-2)4 and Cu2Li2Me4 are the most abundant. It should be noted that species in which the Cu/Li ratio is di erent from 1:1 can also not be ruled out a priori.
Scheme 1.22.
Such equilibria are governed by thermodynamics, and so the abundances of the di erent species in solution are dependent on their relative thermodynamic stabilities. If, however, such a mixture of species is applied in, for example, a conjugate addition reaction, the product formation will be controlled by kinetics, and it is most likely that Cu2Li2Me4 would be kinetically the most active species present.
1.4.2
Anionic Homoleptic and Heteroleptic Organocuprates
The first example of a cuprate structurally characterized by X-ray crystal structure determination was the ionic aggregate [Cu5Ph6][Li(THF)4] [100] (Fig. 1.29A). The structural features of the anionic cuprate unit are closely related to those observed in [Cu4LiPh6] [117] and [Cu3Li2Ph6] [20]. These aggregates have in common that the metal atoms are arranged in trigonal bipyramidal fashion, and the lithium atoms in the latter two compounds reside in axial positions. The six phenyl groups are bridge-bonded, spanning the axial–equatorial edges of the trigonal bipyramid.
Twopeculiarexamplesofanionicaggregatedcupratespeciesare[Cu5Br4(C6H4CHb CH2-2)2] and [Cu5Br2(C6H4CHbCH2-2)4] (Fig. 1.30). In the first compound,

1.4 Organocuprates 33
Fig. 1.29. Structures of [Cu5Ph6] (A) and [Cu3Li2Ph6] (B) in the solid state.
both vinylic substituents are p-coordinated to two adjacent copper atoms, whilst in the second compound only one of the four available vinylic substituents is involved in p-coordination [94]. Bridge-bonding by the phenyl groups, however, as well as various types of Br-to-Cu bridging, is also clearly present in these structures.
Addition of the strongly coordinating 1,2-bis(diphenylphosphino)ethane (DPPE) ligand to a solution of Cu5Mes5 causes a disproportionation reaction, resulting in the formation of ionic [CuMes2][Cu(DPPE)2]. This was the first example of a mononuclear cuprate anion for which the structure was established by X-ray crystal structure determination (Fig. 1.31A) [75]. After this discovery, other ionic mononuclear cuprates were prepared by di erent approaches and structurally characterized. The first approach made use of bulky substituents in the organic groups bound to copper to prevent aggregation. This was achieved in, for example, the crystallization of CuLi[C(SiMe3)3]2 from THF, which gave the ionic compound [Cu(C(SiMe3)3)2][Li(THF)4] [120]. Another approach used an additional ligand
Fig. 1.30. Structures of [Cu5Br4(C6H4CHbCH2-2)2] (A) and [Cu5Br2(C6H4CHbCH2-2)4] (B) in the solid state.

34 1 Structures and Reactivities of Organocopper Compounds
Fig. 1.31. Structures of the mononuclear cuprate anions [CuMes2] (A), [CuPh2] (B), and [Cu(CH(SiMe3)2)Br] (C) in the solid state.
(such as 12-crown-4 or PMDTA (PMDTA ¼ pentamethyldiethylenetriamine)), capable of binding very strongly to the cation; these can break down the aggregated cuprate to form mononuclear ionic species. Examples of mononuclear ionic cuprates obtained in this way are [CuMe2][Li(12-crown-4)2] [121] and [CuPh2][Li(12- crown-4)2] [121] (Fig. 1.31B).
The reaction between equimolar quantities of LiCH(SiMe3)2 and CuBr in the presence of 12-crown-4 a orded [Cu(CH(SiMe3)2)Br][Li(12-crown-4)2], the first example of an ionic mononuclear heteroleptic cuprate [121] for which the structure was established by X-ray crystal structure determination (Fig. 1.31C).
1.4.3
Lowerand Higher-order Cyanocuprates
The importance of cyanocuprates as a synthetic tool in organic chemistry is well established. Depending on the amount of organolithium reagent LiR (one or two equivalents) added to CuCN, two di erent type of cyanocuprates are formed, with stoichiometries of RCu(CN)Li and R2Cu(CN)Li2, respectively [122] (Scheme 1.23). In order to distinguish between these two di erent types of cyanocuprates, the term ‘‘higher-order’’ cyanocuprates was introduced by Lipshutz et. al. for the second type of cyanocuprate, and the term ‘‘lower-order’’ cyanocuprate consequently
Scheme 1.23.

1.4 Organocuprates 35
Fig. 1.32. Proposed structures for higher-order cyanocuprates.
became established for the first type. The earliest report on cyanocuprates (with a 1:1 stoichiometry) dates from 1973 [123].
The discovery of these cyanocuprates and their application in organic synthesis – particularly of the higher-order cyanocuprates, to which a special reactivity was ascribed [97, 124, 125] – resulted in a scientific controversy concerning the actual structure of these compounds. For a number of years, a large number of reports with appealing titles such as ‘‘If the cyano ligand is not on copper, then where is it?’’ [126] and ‘‘It’s on Lithium’’ [127] appeared in the literature. Initially, two models to describe the structure of these cyanocuprates were put forward: (i) a bisanionic species in which two organic groups and the cyanide were bound to the same copper atom (Fig. 1.32A), and (ii) a cyano-Gilman cuprate in which only the two organic groups were bound to copper (Fig. 1.32B). The controversy was resolved in 1999 [128], in favor of proposal B.
For lower-order cyanocuprates, it was already clear at an early stage that the organic group and the cyanide were bound to the same copper atom. An elegant NMR study by Bertz [129] on 13C-labeled MeCu(13CN)Li showed that a coupling of 22 Hz was present between the cyanide carbon atom and the methyl group, which could only be the case if both groups were bound to the same copper atom. This spectroscopic evidence was later confirmed by X-ray crystal structure determinations of [t-BuCu(CN)Li(OEt)2] [130] (Fig. 1.33) and [2,6-Trip2C6H3Cu(CN)Li] (Trip ¼ 2,4,6-(i-C3H7)3C6H3) [131], which appeared in the literature at practically the same time.
It appeared that [t-BuCu(CN)Li(OEt)2] exists in the solid state as a dimer [t-BuCu(CN)Li(OEt)2]2. Two anionic t-BuCu(CN) units, with almost linear geom-
Fig. 1.33. Structure of [t-BuCu(CN)Li(OEt)2]2 in the solid state.

36 1 Structures and Reactivities of Organocopper Compounds
Fig. 1.34. Structure of polymeric [(C6H4CH2NMe2-2)2Cu(CN)Li2(THF)4]n in the solid state.
etries, are linked together through bridging cyanide group nitrogen atoms to two lithium cations. Each lithium cation adopts a tetrahedral coordination geometry as a result of coordination of two diethyl ether molecules. The overall structural features of [2,6-Trip2C6H3Cu(CN)Li(OEt)2]2 are very similar to those of [t- BuCu(CN)Li(OEt)2]2.
NMR investigations [129, 132, 133], EXAFS and XANES studies [134–136], and theoretical calculations [127, 137, 138] performed on higher-order cyanocuprates strongly suggested that the cyanide anion was not bound to copper in these R2Cu(CN)Li2 species. Additional evidence was provided by the first X-ray crystal structure determinations of ‘‘higher-order’’ cyanocuprates: [(C6H4CH2NMe2-2)2 Cu(CN)Li2] [139] (Fig. 1.34) and [(tBu)2Cu(CN)Li2] [130] (Fig. 1.35).
The molecular structure of the first compound comprises a polymeric chain, consisting of alternating [(C6H4CH2NMe2-2)2Cu] anionic and [Li2(CN)(THF)4] cationic units. In the cationic unit, two lithium atoms are end-on bridged by the cyanide group, and two additional THF molecules are coordinated to each lithium atom. The fourth coordination site is occupied by the nitrogen atom of the adjacent (dimethylamino)methylphenyl group of the [(C6H4CH2NMe2-2)2Cu] anionic unit.
On the basis of molecular weight determinations by cryoscopy in THF and conductivity measurements, it was concluded that the polymeric chain breaks up in solution to form smaller aggregates, probably giving rise to solvent-separated
Fig. 1.35. Structure of [t-Bu2Cu][Li2CN(THF)2(PMDTA)2] in the solid state.

1.5 Concluding Remarks 37
ion-pairs. The presence of donor solvents, THF in this case, may greatly contribute to the (thermodynamic) stability of a particular structure. This may be inferred from the observation that the use of a less polar solvent such as benzene induces a disproportionation reaction, giving the neutral homoleptic cuprate [Cu2Li2(C6H4CH2NMe2-2)4] (discussed previously) and LiCN.
The structure of [t-Bu2Cu(CN)Li2] in the solid state consists of isolated [t- Bu2Cu] anionic units and [Li2CN(THF)2(PMDTA)2] cationic units (Fig. 1.35). The structural features of the linear RaCuaR arrangement are identical to those observed for other [R2Cu] anionic units discussed previously (cf. Fig. 1.31). The [Li2CN(THF)2(PMDTA)2] cationic unit consists of a central cyanide moiety, to which two lithium atoms are bound in end-on fashion. Coordination saturation at each lithium atom is achieved by coordination of the three nitrogen atoms of the PMDTA molecule and one THF molecule, rendering each lithium atom pentacoordinate. Recent 1H, 6Li HOESY experiments showed that this ionic structure found in the solid state is probably retained in polar solvents such as THF [140].
The solution structures of cyano-Gilman cuprates and lower-order cyanocuprates have been studied by cryoscopic measurements in THF [141]. The results of this study have in several cases shown ways to obtain useful single crystals of several higherand lower-order cyanocuprates and consequently to determine their structures in the solid state. It appears that a number of these cyanocuprates retain their observed solid-state structure when dissolved in THF.
1.5
Concluding Remarks
This review substantiates the earlier opinion [29, 45] that the various types of organocopper compounds known today are almost always highly aggregated species. In spite of this structural information, it remains very di cult (and often also incorrect) to correlate a given structural feature of an organocopper or cuprate reagent with its specific reactivity. It always has to be kept in mind that X-ray crystal structural information is generally obtained from crystalline material that selectively crystallized out of a solution existing as a complicated equilibrium mixture of a number of aggregates, rather than as a solution of one pure compound. The aggregate that crystallizes from solution is the thermodynamically most stable one, and this is often the kinetically less reactive species. Indeed, there are only a very few examples of compounds for which it has been proven that the structure as observed in the solid state is retained in solution. One such is [Cu2Li2(C6H4CH2NMe2-2)4] in apolar solvents such as benzene.
Another factor that complicates understanding of reaction mechanisms and of the actual species involved in reactions of organocopper compounds is the strong tendency of organocopper compounds and cuprates to aggregate with metal halides. These metal halides are often formed as unavoidable co-products when an organocopper compound or an organocuprate is applied as a reagent in organic synthesis. This means that, during a reaction, the initial reagent is gradually con-
