

22. Nitric oxide from arginine: a biological surprise |
983 |
as the cofactor for phenylalanine hydroxylation to tyrosine80 as well as tyrosine and tryptophan hydroxylation81. Kwon and coworkers37 not only found that the production of NO was stimulated, but found that a reduction of the pterin could be accomplished if FAD and GSH were added. Therefore, they included in their article the picture shown in Figure 3 to illustrate the reaction.
Actually, Tayeh and Marletta36 had also found BH4 to be a cofactor for mouse macrophages one month previously but had emphasized the NO synthesis. In the picture that they had included, the first hydroxylated arginine was shown as N-hydroxyl-L-arginine although this compound had not yet been published. The reactions leading to NO and citrulline are shown in Figure 4.
The synthesis of NG-hydroxy-L-arginine was published by Pufahl and Marletta82. This compound was shown to be a substrate for the production of nitrite and nitrate, for the production of NO and for the synthesis of citrulline. When 15N-NHA was used as substrate, the NO2 /NO3 produced were found to contain undiluted 15N. Therefore, they concluded that the NG-hydroxy-L-arginine is a true substrate in NO synthesis.
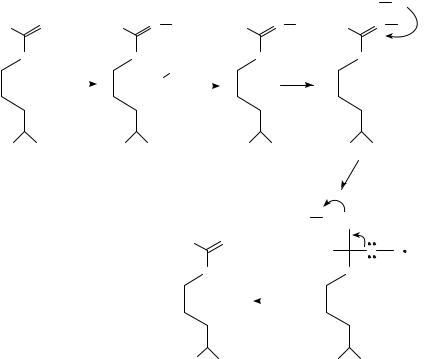
Hibbs and coworkers34 had reported that the NG-methyl-L-argine was a potent inhibitor of macrophage nitrite generation. This was subsequently studied by Olken and collaborators83. They found that NG-methyl-L-arginine is indeed an inhibitor with a Ki approximately equal to the Km of arginine (4 2 mM for the methyl compound and 7.4 mM for arginine). Two of the mechanisms of the inhibitor are illustrated in Figure 5. They also included a third mechanism of a peroxide formation. The lower case given, in which a Michael acceptor is shown, seems unlikely since the authors state that this should give formaldehyde and L-arginine, which should overcome the inhibition.
V. COMPLEXITIES OF NOS
A. Role of BH4
The role of BH4 was made contentious by the finding that it is not recycled in the brain NOS. Giovanelli’s group84 used the enzyme prepared according to Bredt and Snyder43 and showed that the protein responded to BH4. The results of Giovanelli’s group84 showed that the BH4 acts in very low concentration (<1.0 mm) and that it does not have to be reactivated during the catalysis. Each BH4 molecule is responsible for >15 moles of product. They conclude that the function may be allosteric or it may serve to maintain some groups in a reduced state required for activity.
The above conclusion84 was negated by the findings of Hevel and Marletta85. These authors used mouse macrophages to study the effect of BH4 on NOS. With the purification used in the past, they found only a small portion of the BH4 relative to other cofactors and in this case they found a considerable activation on adding more BH4 to the reaction. However, when 5ðM BH4 was added to the solutions used for preparation of the enzyme, they found essentially a 1:1 ratio of BH4 to the enzyme and no effect of adding more BH4. They concluded that BH4 is used in an oxidative reaction of the macrophage NOS. Of course, a possibility as to the difference between the results of Giovanelli’s’ group89 and of Hevel and Marletta85 is the use of bNOS and iNOS, respectively.
B. Iron in NOS
Iron was first found in NO synthase by Mayer and coworkers86. Probably because the amount of enzyme obtained from brain was small, they reported the iron as non-heme. The next year, however, the iron was reported by White and Marletta87 as heme iron. The heme was identified at a mixture of high-spin and low-spin states with a shoulder at

984 |
Alan H. Mehler |
NADPH
O2
Fe+++
H2 N NOH
NH
+
H3 N COO−
FIGURE 6
NADP+
H2 O
(FeO)+++
H2 N NH2
N
+
H3 N COO−
406 nm, previously shown by Nardi and Fulco88 to be due to FMN and FAD bound to the enzyme. White and Marletta87 proposed that the iron might work as a carrier of oxygen and be responsible for the formation of NG-hydroxy-L-arginine. They also demonstrated that the porphyrin was protoporphyrin IX and presented the CO spectrum. The work was with mouse macrophage NOS and a partially purified bNOS was said to have similar properties. This is shown in Figure 6.
In an article published independently at the same time, Stuehr and Ikeda-Saito89 used the purified bNOS and iNOS to reach the same conclusions. While the paper was under review, the authors mention that White and Marletta had reported earlier that the iron was a heme and was used as an oxidant. Using the same type of study, they found that the iron prophyrin and its CO derivative had the expected properties and proposed that the iron is penta-coordinated, with a cysteine thiolate as the fifth coordinate. A third publication confirmed the results when McMillan and coworkers90 used bNOS grown in human kidney cells. These workers obtained similar data for the light absorption of the enzyme and its CO spectrum. They also speculate on very similar sequences in the three types of purified enzyme that might be the porphyrin binding site.
Mayer and collaborators45 also found biopterin in NOS and found iron, which they claimed was non-heme. The biopterin they postulated was reduced by NADPH. Therefore, they proposed the mechanism shown in Figure 7 to account for the overall reaction.
|
22. Nitric oxide from arginine: a biological surprise |
|
985 |
||||||||||||
|
|
|
|
|
|
|
NH |
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
C |
L-arginine |
|
|
|
|
||||
|
|
|
|
|
|
|
NH2 |
|
|
|
|
|
|
|
|
|
NADP+ |
|
H Biopterin |
|
|
|
|
O2 |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
4 |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
(Fe) |
|
|
|
|
|
|
|
|
|
|
|
H++ NADPH |
|
9-H2 Biopterin |
|
|
|
|
H2 O |
|
|
|
|
|||
|
|
|
|
|
|
NOH |
|
|
|
|
|
|
|
||
NADP+ |
FADH2 |
O2 |
O2 |
R |
C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NH2 |
|
|
|
|
O |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
R |
C |
L-Citrulline |
||
|
FADH |
|
HO2 |
|
|
|
|
|
|
|
|
|
NH2 |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
O2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NO. |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
H2 O |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H++ NADPH |
|
|
|
|
|
N O |
|
|
|
O |
O H |
|
|
||
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
||||||
FAD |
|
HO2 |
+ R |
C |
H |
|
|
|
R C |
H |
|
|
|
||
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
NH2 |
|
|
|
NH2 |
|
|
|
||
H2 O2
FIGURE 7
Stuehr and coworkers91 synthesized NG-hydroxy-L-arginine and suggested the scheme shown in Figure 8 to make up the action of NOS.
Using bNOS, Klatts’ group92 determined that the BH4 gave five times the rate of NADPH and that Fe was part of the enzyme. Without deciding where the BH4 reacted, they proposed the scheme shown in Figure 9.
Pufahl and Marletta82, with the NG-hydroxy-L-arginine that they had synthesized, gave the outline of the scheme presented in Figure 10.
Using a spectrophotometric method, McMillan and Masters93 obtained evidence that bNOS contained a heme group that reacted with L-arginine and with NG-hydroxy-L- arginine (an intermediate) and NG-methyl-L-arginine (an inhibitor). They therefore concluded that the heme was an oxygen donor.
In a minireview, Marletta94 postulated the series of reactions in Figure 11. The reactions show the details of the iron porphyrin reaction and are concerned with the formation of NO.
In a review without references, Feldman and coworkers95 proposed a very similar scheme to Marletta94 but with the nitrogen of hydroxyarginine assuming an iminoxyl radical that is converted to NO. Korth’s group96 accept the scheme published by Marletta94 but proposed that the NG-hydroxy-L-arginine must be oxidized according to the mechanism of Figure 12. This sequence of reactions argues against the participation of the iminoxyl radical of NOH as the reductant because it is likely to lose a proton more rapidly, and therefore reactions A, B, F, G, H and I are proposed.
Using a purified bNOS, Campos and collaborators97 studied the hydroxylation of L- arginine by the enzyme minus NADPH. They found 0.16 mole of NOH per mol of NOS. The presence of reducing agents in the purified bNOS was measured as much less than the NOH formed. Possible reagents that could have been responsible are flavin and BH4.
986 |
Alan H. Mehler |
NADPH
L-arginine ! N-hydroxy-L-arginine
O2
FIGURE 8
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NH2 |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RN |
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
O |
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
NH2 |
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
Fe3 + |
|||
RN |
|
|
C |
|
N |
|
O + Fe3 |
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
|
|
|
|
|
|
|
|
|
|
|
H++ |
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NO |
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
NH2 |
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O)3 + |
|
|
|
|
|
|
||||||||
RNH |
|
|
C |
|
N |
|
O (Fe |
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
H2 O |
|
|
|
|
|
|
|
|
|
H2 O2 , O2 |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
2H+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
NH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
+ |
|
|
|
|
|
− |
|
− |
||||||||
RNH |
|
|
C |
|
N |
|
O Fe2 |
|
|
O2 |
|
O2 |
||||||||||||||||||
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
e− |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
NH2 |
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
RNH |
|
|
C |
|
N |
|
O Fe2 |
|
|
O2 |
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
O2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
NH2 |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
RNH |
|
|
C |
|
N |
|
O Fe2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
H+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Fe3 + |
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
NH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
RNH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
C |
|
|
N |
|
|
OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.5 NADPH
! NOž C citrulline
O2
BH4
+
NH3
HN |
|
C |
|
|
|
NHR |
|
|
NH3 |
|
|||||
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
Fe3 + HN |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
C |
|
NHR |
|||||
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
e− |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
NH3 |
|
||
|
|
|
|
Fe2 + + ΗΝ |
|
|
|
|
|||||||
|
|
|
|
|
C |
|
NHR |
||||||||
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
O2 |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
NH3 |
|
||
|
O2 |
|
|
|
|
Fe2 + ΗΝ |
|
|
|
|
NHR |
||||
|
|
|
|
|
C |
|
|||||||||
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
||
|
− |
|
|
|
|
|
|
|
|
NH3 |
|
||||
|
|
|
|
|
Fe3 + ΗΝ |
|
|
|
|
|
|
||||
|
O2 |
|
|
|
|
|
|
C |
|
NHR |
|||||
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
e− |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|||
|
− |
|
|
|
|
|
|
|
|
NH3 |
|
||||
|
|
|
|
|
Fe2 + ΗΝ |
|
|
|
|
|
|
||||
|
O2 |
|
|
|
|
|
|
C |
|
NHR |
|||||
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
2H+ |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
H2 O |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
NH3 |
|
||
|
(O |
|
|
|
Fe)3 + ΗΝ |
|
|
|
|
NHR |
|||||
|
|
|
C |
|
|||||||||||
|
|
|
|
|
|
|
|||||||||
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|||||||||
FIGURE 9
|
|
|
|
22. Nitric oxide from arginine: a biological surprise |
|
|
|
|
987 |
|||||||||||
|
|
|
|
|
NADPH |
|
|
|
NADPH |
|
|
|
|
|
||||||
|
|
L-arginine ! NG-Hydroxy-L-arginine ! citrulline |
C |
NO |
|
|||||||||||||||
|
|
|
|
|
O2 |
|
|
|
O2 |
|
|
|
|
|
|
|
||||
FIGURE 10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PPIX |
|
|
Fe+ + + |
|
PPIX Fe+ + + |
|
PPIX Fe+ + Ο2 |
|
|
PPIX Fe+ + + OO− |
||||||||||
|
|
|
|
|
|
|||||||||||||||
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
H2 N |
|
NH2 |
|
H2 N |
N OH |
H2 N |
N OH H2 N |
N OH |
||||||||||||
|
|
NH |
|
|
NH |
|
|
NH |
|
|
|
|
|
|
|
|
NH |
|||
|
|
|
|
NA DPH |
|
|
1 |
NA DPH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
O2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
COO− |
+ |
COO− |
+ |
|
COO− |
|
|
+ |
COO− |
||||||||
H3 N |
|
|
H3 N |
H3 N |
H3 N |
|||||||||||||||
|
|
|
|
|
|
|
NHA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PPIX Fe+ + + |
PPIX Fe+ + + |
O |
|
|
O |
|
|||||
|
|
|
|
|
|
|
|
|
H2 N |
O |
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
H2 N |
|
|
N O |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
• NO + |
|
|
|
|
|
|
|
|
|
|
|
− |
|
|
|
|
|
|
|
|
NH |
|
|
|
|
|
|
|
|
|
NH |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H N COO− |
H |
+ |
COO− |
N |
|||
3 |
3 |
|
|
FIGURE 11
C. Subunits and Dimers
Although the first purification of bNOS was a monomer, it is now clear that the enzyme in all cases is effective as a dimer. A purified macrophage iNOS was used by Baek and coworkers98 to separate the holoenzyme from the monomers. The subunits do not have NOS activity but do have the ability to oxidize reduced triphosphopyridine nucleotide with either ferricyanide, cytochrome c or dichlorophenolindophenol. When all of the missing factors are present, but not when any is missing, the authors find recombination, as shown in Figure 13.
Ghosh and Stuehr99 found the two subunits combined in a head-to-head fashion. They consider that the two tails could be free or could be somehow bound.
Raman spectra were performed by Wang’s group100. They found the heme to have a 5-coordinate high spin configuration. The fifth ligand was an axial bond to the thiolate, which was confirmed by the Fe C O bending mode at 562 cm 1.

988 |
|
|
|
|
|
|
|
Alan H. Mehler |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
NA DPH |
|
|
O2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
PPIX Fe+ + + |
2 |
|
PPIX Fe+ + |
|
PPIX Fe+ + +OO |
|
|
|
|
|
|
|
|
|
|
|
||||||
|
− 1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
NA DP |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
A |
|
|
|
B |
|
PPIX Fe+ + + OOH |
|
|
|
PPIX Fe+ + + |
||||||||
|
|
|
|
+ PPFe+++OO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|||
|
|
|
|
|
|
|
|
F |
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
+ |
|
+ |
|
O• |
|
|
|
|
O |
|
O |
|||||
H3 N |
NOH |
C |
H3 N |
N OH D |
|
H3 N |
N |
|
|
|
H3 N |
|
N |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
NH |
|
|
|
|
|
|
|
NH |
|
|
NH |
G |
|
|
|
|
NH |
|
|
|
||
|
|
|
+ PPIX Fe+++OO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
− PPIX Fe+++OO − |
|
|
|
|
− H + |
|
|
|
|
|
|
|
|
|
|
|
|
||
H3 N |
COO− |
|
|
|
+ |
COO− |
+ |
COO− |
|
|
|
+ |
COO− |
|||||||||
|
|
H3 N |
|
H3 N |
|
|
|
H3 N |
||||||||||||||
|
|
|
|
|
|
|
|
+ PPIX Fe+++ − OO − |
E |
|
H |
|
− H + |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
H2 N |
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NH |
|
|
|
|
|
|
|
PPFe+ + + |
OH |
||
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
O |
|
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
+ H |
+ |
− H2 O |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
PPIX Fe+ + + |
||||
|
|
|
|
|
|
|
|
|
|
COO− |
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
H3 N |
|
|
|
|
|
|
|
|
|
|
||
FIGURE 12 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
N |
|
|
CAM |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FAD |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
Fe |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FMN |
|
|
|
||
|
|
|
|
N |
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
FMN |
|
|
|
H4B |
H4B |
FMN |
Arginine |
2 |
|
|
|
|
|
FAD |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
FAD |
|
|
|
N |
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fe |
|
|
|
|
|
|
|
|
|
CAM |
|
|
|
|
|
|||
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
CAM |
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+2 |
|
|
|
N |
N |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fe |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
N |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
+2 |
|
|
|
H4B |
|
|
|
|||
FIGURE 13 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
22. Nitric oxide from arginine: a biological surprise |
989 |
The activity of bNOS is firmly bound to the amino acids that comprise the enzyme and is able to withstand separation of the two halves by tryptic cleavage. Sheta and collaborators101 separated ca 79 and ca 89 fractions from bNOS and found that one fraction (ca 79) had the heme and the other (ca 89) had the flavin and was able to reduce cytochrome c. When the two domains of the enzyme were held together with calmodulin, the appearance of the two domains was prevented. The finding of retained activity of the two halves was confirmed by McMillan and Masters102, who produced the heme component and the flavin-binding component with and without the calmodulin site as proteins produced in E. coli. The workers showed the heme had spectral properties of NOS and that the fifth ligand was Cys415 by changing this to histidine in a site-specific mutation: the mutant did not bind heme. Chen and coworkers103 prepared mutants of the eNOS and grew them in COS-1 and baculovirus cells. Alanine instead of cysteine in positions 235 and 441 retained the heme and NOS activity of the enzyme, but these changes at cysteine 99 and 241 gave no NOS activity. The authors picked Cys184 as being responsible, because the peaks with CO were missing whereas the Cys99 still gave the CO change. At the same time that Chen’s group103 reported the above, Richards and Marletta104 reported the findings with neural NOS and the C415H mutant. Heme at 7 ð M gave a 7-fold greater activity and there was about a 50% increase with BH4 in the assay. The C415H mutation gave no NOS activity and the enzyme was devoid of heme. The flavin spectra in the region 450 to 500 mm were normal. This was the first evidence that the Cys 415 binds the heme.
D. Complications of Many Cofactors
By resonance Raman methods, Wang and coworkers105 showed NO bound to both ferric and ferrous heme of bNOS. Hurshman and Marletta106 used iNOS and spectrophotometric methods to show similar reactions, although the physiological effects will depend on the effects of arginine and oxygen in vivo.
Using neuronal NOS, Matsuoka and collaborators107 observed that arginine apparently reacted with the heme to reduce the rate of CN or CO reacting. The reaction of L-arginine
1 |
CAM |
|
2 |
O2 |
|
NADPH
e− |
|
O2 − |
|
|
|||
|
|||
FAD, FMN |
e− |
||
|
heme Fe+ + + |
||
|
|
|
Arginine |
|
|
|
NO |
|
|
|
|
cytochrome c
Fe(CN)6
FIGURE 14
990 |
Alan H. Mehler |
is shown to take place with the iron instead of the H2O. Calmodulin does not affect the iron but permits electrons to flow from the flavins.
NO in the concentrations formed by NOS from brain were shown by Griscavage and coworkers108 to inhibit the synthase about 1,000ð more than CN . This effect was attributed to binding to the ferric heme and could be reversed by BH4 but not by other reducing agents. Mayer’s group109 used a Clark-type nitric oxide electrode to follow the reaction of bNOS and found also a strong inhibition by BH4. They conclude that BH4 reacts with SOD to peroxynitrite.
Abu-Soud and coworkers110 were able to remove heme and BH4 from NOS by dialysis with 2 M urea. The remaining enzyme retained the ability to reduce ferricyanide and cytochrome c. The reduction of cytochrome c was about 9ð in the presence of calmodulin. The two sites of calmodulin for influencing the reaction are shown in Figure 14.
Hobbs and coworkers111 used a specific chemiluminescent reaction to measure NO. With this they determined that the reaction measured by citrulline formation was not affected by SOD but that NO was increased.
E. Inhibitors of NOS
A further study by Olken and collaborators112 describes inactivation of mouse iNOS by NG-methyl-L-arginine. The inactivation occurs only in the presence of oxygen. Only a small amount of 3H or 14C label from the labeled methyl group of NG-methyl-L-arginine
PPIX |
|
Fe+++ |
PPIX |
|
|
Fe+++ |
|
OO− |
PPIX |
|
|
Fe+++ |
|
O |
|
O |
|||||||||||
|
|
|
|
|
|
|
|
||||||||||||||||||||
H2 N |
NOH |
H2 N |
|
|
|
NOH |
|
|
|
|
|
|
H2 N |
N OH |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
− |
NH |
|
|
|
|
|
|
|
|
NH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NH |
|||
|
|
|
HOOH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
− |
+ |
|
|
|
|
|
− |
|
|
|
|
+ |
− |
||||||||||||
H3 N |
COO |
H3 N |
|
|
|
|
COO |
|
|
|
|
|
|
H3 N |
COO |
||||||||||||
L-NHA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
Ferric nitroxyl PPIX |
|
|
Fe+++ |
|
NO− |
|
|
PPIX |
|
|
Fe+++ + NO − |
||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H2 N |
|
O |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
Ferrous nitrosyl PPIX |
|
Fe+++ |
|
NO |
|
|
|
|
NH |
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
|
|
λmax440, 578 nm |
|
|
|
|
|
|
|
O2 |
(aerobic) |
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HOOH |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
NO |
− |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
2 |
NO |
− |
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
− |
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H3 N |
|
|
COO |
|
|||||
FIGURE 15
|
22. Nitric oxide from arginine: a biological surprise |
991 |
||||||||||||||
NADPH |
e− |
FAD, FMN |
|
|
|
|
heme Fe |
+ + + |
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
− CAM |
|
+ CAM |
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
O2 |
|
Arginine |
||
|
|
|
|
CAM |
|
|
|
|
|
|
||||||
NADPH |
e− |
FAD, FMN |
|
e |
− |
|
heme Fe+ + + |
|
|
|
||||||
|
|
|
|
|
|
|
|
O |
− |
NO |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
Thiocitrulline |
|
|
e− |
2 |
||||||||
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
NAME |
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
Dithionite |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
FIGURE 16
was included, showing that the reaction is varied. A large amount of the heme was lost in inactivation. Simultaneous studies by Abu-Soud group113 with three inhibitors, Nω -methyl-L-arginine, Nω -nitro-L-arginine methyl ester and thiocitrulline, affected both neuronal and macrophage NOS. Whereas the Nω -methyl-L-arginine was similar to L- arginine in that it did not prevent the reduction of heme, both Nω -nitro-L-arginine and thiocitrulline did. The inhibition by both of these compounds seems to block at two points, shown in Figure 15.
A variety of L-arginine-based inhibitors was tested by Komori and coworkers114. They found NG-methoxy-L-arginine as well as NG-hydroxy-L-arginine and L-arginine itself to oxidize NADPH. The mechanism of the effects remain to be determined.
The ability of murine macrophage NOS to use peroxides in place of oxygen was studied by Pufahl and collaborators115. Cumene hydroperoxide and tert-butylhydroperoxide were inactive but hydrogen peroxide supported product formation. Interestingly, L-arginine was not used but NG-hydroxy-L-argine was a substrate. The authors proposed the mechanism for the hydrogen peroxide in Figure 16.
Several specific inhibitors of NO synthetases have been found. The species of all three human NOS was found by Garvey’s group116 to be inhibited by isothioureas, with ethylthioruea the most powerful. Furfine and coworkers117 found S-methyl-L-thiocitrulline and S-ethyl-L-thiocitrulline were effective vs neuro NOS in human, but there seems to be difficulty in in vivo studies. Narayanan’s group118 reported on several S-alkyl-L-thiocitrullines from rat with nNOS and iNOS. The methyl compound is most potent and is reversible. Another type of inhibitor was found by Wolff and Griben119 to be imidazole and its phenyl derivatives and120 also substituted nitroindazole, especially the 7-nitroindazole. Wolff and Lubeskie121 reported that aminoguanidine is a mechanism-based inhibitor of the three types of NOS.
F. Factors for Growth of NOS
Hartneck and coworkers122 grew rat bNOS in baculovirus. Calcasi and collaborators124 found that the bNOS gives a burst of superoxide when stimulated by N-methyl-D-aspartate. This is arachadonic-independent and is suppressed by L-arginine or NG-nitro-L-arginine. The superoxide gives cell death.
Xie’s group123 measured two closely linked but separable promotors for iNOS. Lowenstein and coworkers125 reported that bacterial lipopolysaccharide (LPS) promotes iNOS
992 |
Alan H. Mehler |
and that IFNdoes not by itself but with LPS gives a big stimulation. This was confirmed by Weisz and coworkers126. Balligand’s group127 detected the iNOS in single myocytes. Two distinct pathways for generating expression of iNOS in rat cells were demonstrated by Kunz and coworkers128. Xie and collaborators129 reported that NF- B is essential for LPS induction of iNOS in the mouse. They found two NF- B, one in the 50 -region, NFBu, and one in the downstream location, NFBd. The downstream NFBu is responsible for the induction of LPS. Chu’s group130 described a large series of human iNOS that varied in the initial codons, many lacking the first exon.
Xie and coworkers131 cloned the NOS from RAW264.7 cells and found two sets of cDNA. The smaller set was 22 amino acids shorter. They found that this cDNA did not produce NOS. By removing amino acids, they found Phe reduced the level to 41%, removing the Ile1121 further reduced the level to 95% and further removal lost all NOS activity. This is the first example of the importance of the COO region.
The eNOS has been purified from many tissues. Human NOS was purified from placenta by Garvey collaborators132. Balligand’s group133 found rat eNOS in myocytes and showed it to be responsible for cholinergic and ˇ-adrenergic regulation and muscular contraction. The eNOS of guinea pigs was shown to be dependent on sex hormones by Weiner and coworkers134. This was the first evidence that the eNOS was inducible. A human colon adenocarcinoma was found by Jenkins and collaborators135 to be stimulated by eNOS.
Schmidt’s group136 reported that two of the arginine derivatives inhibit NOS, NG- methyl-L-arginine and NG-nitro-L-arginine, and are taken up by cationic amino acid transporter and neutral amino acid transporter of macrophages. The same authors137 found that peroxynitrite produces the same change in hemoglobin as NO, but that it does not interfere with the Clark-type determination of NO. Peroxynitrite was also found to be the compound that inactivates aconitase. In a couple of papers published together in J. Biol. Chem., Hausladen and Fridovich138 and Castro’s group139 reported that this ironcontaining enzyme is not inhibited by NO but is inhibited by peroxynitrite. Hecker and coworkers140 described an adduct of NG-hydroxyl-L-arginine with NO as the product made by IL-1ˇ in rat muscle that yields NO immediately with NOS.
VIII. PHYSIOLOGICAL FUNCTIONS OF NOS
The two most surprising aspects of NO are the large number of alterations of metabolism controlled by a simple gas and the most extensive list of diseases that may be caused by either an increase or decrease in the concentration of the molecule. The second function, the clinical function of NO, has occupied the attention of both the preclinical and clinical investigators and is responsible for the growth in the literature. To attempt to cover the clinical literature is far beyond the scope of this review but the outlines of the subject cannot be ignored. The scope of this branch of the literature is expanding while this chapter is being written, and the impact of NO on medicine is just arriving.
The early studies on NOS in macrophages suggested to the authors that the synthesis of NO was responsible for the killing of tumor cells and certain pathogens28. It is now clear that the application of nitroglycerin and related compounds is due to the release of NO141 and that eNOS carries out this function in vivo. However, NO also is responsible for killing neuronal cells. Zhang and coworkers142 have determined that NO was capable of damaging the DNA of sensitive cells and, by activating poly (ADP-ribose) synthetase, kill the cells by energy depletion.
The finding of a brain NOS42,43 led to speculation that various neurological disorders were due to this molecule. Therefore, it was of great significance when Huang and collaborators143 produced a strain of mice completely lacking bNOS. These mice had
